Photographic fixer is a mix of chemicals used in the final step in the photographic processing of film or paper. The fixer stabilises the image, removing the unexposed silver halide remaining on the photographic film or photographic paper, leaving behind the reduced metallic silver that forms the image. By fixation, the film or paper is insensitive to further action by light. Without fixing, the remaining silver halide would darken and cause fogging of the image. Fixation is commonly achieved by treating the film or paper with a solution of thiosulfate salt. Popular salts are sodium thiosulfate—commonly called hypo—and ammonium thiosulfate—commonly used in modern rapid fixer formulae. Fixation involves these chemical reactions (X = halide, typically Br−):

Sodium thiosulfate is an inorganic compound with the formula Na2S2O3·xH2O, where x indicates the number of water molecules in the compound. Typically it is available as the white or colorless pentahydrate, Na2S2O3·5H2O. The solid is an efflorescent crystalline substance that dissolves well in water.

Thiosulfate is an oxyanion of sulfur with the chemical formula S2O2−3. Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, e.g. sodium thiosulfate Na2S2O3. The prefix thio- indicates that the thiosulfate is a sulfate with one oxygen replaced by sulfur. Thiosulfate is tetrahedral at the central S atom. Thiosulfate salts occur naturally. Thiosulfate ion has C3v symmetry, and is produced by certain biochemical processes. It rapidly dechlorinates water and is notable for its use to halt bleaching in the paper-making industry. Thiosulfate salts are mainly used in dying in textiles and the bleaching of natural substances.

Rhodanese, also known as rhodanase, thiosulfate sulfurtransferase, thiosulfate cyanide transsulfurase, and thiosulfate thiotransferase, is a mitochondrial enzyme that detoxifies cyanide (CN−) by converting it to thiocyanate (SCN−).
In enzymology, a pyrroloquinoline-quinone synthase (EC 1.3.3.11) is an enzyme that catalyzes the chemical reaction
In enzymology, a glucose 1-dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a malate dehydrogenase (quinone) (EC 1.1.5.4), formerly malate dehydrogenase (acceptor) (EC 1.1.99.16), is an enzyme that catalyzes the chemical reaction
In enzymology, a quinoprotein glucose dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a phenylacetyl-CoA dehydrogenase (EC 1.17.5.1) is an enzyme that catalyzes the chemical reaction

In enzymology, a ribosyldihydronicotinamide dehydrogenase (quinone) (EC 1.10.99.2) is an enzyme that catalyzes the chemical reaction
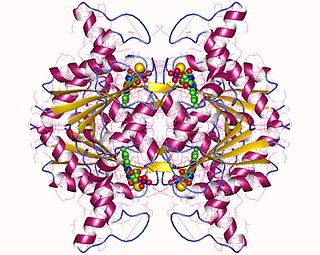
In enzymology, a NAD(P)H dehydrogenase (quinone) (EC 1.6.5.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a NADPH dehydrogenase (quinone) (EC 1.6.5.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a NADPH:quinone reductase (EC 1.6.5.5) is an enzyme that catalyzes the chemical reaction

Thiosulfate dehydrogenase is an enzyme that catalyzes the chemical reaction:
In enzymology, a trithionate hydrolase (EC 3.12.1.1) is an enzyme that catalyzes the chemical reaction
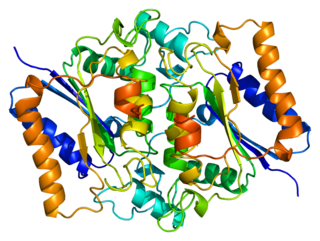
NAD(P)H dehydrogenase, quinone 2, also known as QR2, is a protein that in humans is encoded by the NQO2 gene. It is a phase II detoxification enzyme which can carry out two or four electron reductions of quinones. Its mechanism of reduction is through a ping-pong mechanism involving its FAD cofactor. Initially in a reductive phase NQO2 binds to reduced dihydronicotinamide riboside (NRH) electron donor, and mediates a hydride transfer from NRH to FAD. Then, in an oxidative phase, NQO2 binds to its quinone substrate and reduces the quinone to a dihydroquinone. Besides the two catalytic FAD, NQO2 also has two zinc ions. It is not clear whether the metal has a catalytic role. NQO2 is a paralog of NQO1
NQO2 is a homodimer. NQO2 can be inhibited by resveratrol. One of QR2's binding sites responds to 2-iodomelatonin, and has been referred to as MT3.

NADH:ubiquinone reductase (non-electrogenic) (EC 1.6.5.9, NDH-2, ubiquinone reductase, coenzyme Q reductase, dihydronicotinamide adenine dinucleotide-coenzyme Q reductase, DPNH-coenzyme Q reductase, DPNH-ubiquinone reductase, NADH-coenzyme Q oxidoreductase, NADH-coenzyme Q reductase, NADH-CoQ oxidoreductase, NADH-CoQ reductase) is an enzyme with systematic name NADH:ubiquinone oxidoreductase. This enzyme catalyses the following chemical reaction:
Sulfide:quinone reductase is an enzyme with systematic name sulfide:quinone oxidoreductase. This enzyme catalyses the following chemical reaction

Acidithiobacillus thiooxidans, formerly known as Thiobacillus thiooxidans until its reclassification into the newly designated genus Acidithiobacillus of the Acidithiobacillia subclass of Pseudomonadota, is a Gram-negative, rod-shaped bacterium that uses sulfur as its primary energy source. It is mesophilic, with a temperature optimum of 28 °C. This bacterium is commonly found in soil, sewer pipes, and cave biofilms called snottites. A. thiooxidans is used in the mining technique known as bioleaching, where metals are extracted from their ores through the action of microbes.

Trithionate is an oxyanion of sulfur with the chemical formula S
3O2−
6. It is the conjugate base of trithionic acid. Dilute sodium hydroxide hydrolyzes S
4N
4 as follows, yielding sodium thiosulfate and sodium trithionate:










