
Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl. It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations and hypochlorite anions.
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for human consumption, but water purification may also be carried out for a variety of other purposes, including medical, pharmacological, chemical, and industrial applications. The history of water purification includes a wide variety of methods. The methods used include physical processes such as filtration, sedimentation, and distillation; biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination; and the use of electromagnetic radiation such as ultraviolet light.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 has an α-polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

Hypochlorous acid is an inorganic compound with the chemical formula ClOH, also written as HClO, HOCl, or ClHO. Its structure is H−O−Cl. It is an acid that forms when chlorine dissolves in water, and itself partially dissociates, forming a hypochlorite anion, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

In chemistry, hypochlorite, or chloroxide is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite and calcium hypochlorite. The Cl-O distance in ClO− is 1.69 Å.

Cyanuric acid or 1,3,5-triazine-2,4,6-triol is a chemical compound with the formula (CNOH)3. Like many industrially useful chemicals, this triazine has many synonyms. This white, odorless solid finds use as a precursor or a component of bleaches, disinfectants, and herbicides. In 1997, worldwide production was 160 000 tonnes.

Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant.
Calcium hypochlorite is an inorganic compound with chemical formula Ca(ClO)2, also written as Ca(OCl)2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.

Sodium dichloroisocyanurate is a chemical compound widely used as a cleansing agent and disinfectant. It is a colorless, water-soluble solid, produced as a result of reaction of cyanuric acid with chlorine. The dihydrate is also known as is the potassium salt.
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting point of −66 °C (−87 °F), but it is usually handled as a dilute aqueous solution, in which form it is sometimes used as a disinfectant. Chloramine is too unstable to have its boiling point measured.
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N−H bonds have been replaced by N−Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines. Chloramines are the most widely used members of the halamines.
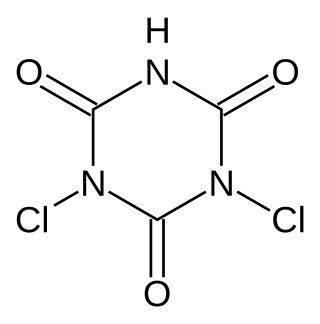
Dichloroisocyanuric acid, also known as dichlor or dichloro-s-triazinetrione and is marketed under many names (e.g. troclosene), is a chemical compound with the formula (C(O)NCl)2(C(O)NH).

Swimming pool sanitation is the process of ensuring healthy conditions in swimming pools. Proper sanitation is needed to maintain the visual clarity of water and to prevent the transmission of infectious waterborne diseases.
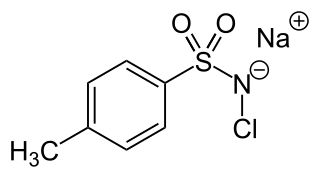
Chloramine-T is the organic compound with the formula CH3C6H4SO2NClNa. Both the anhydrous salt and its trihydrate are known. Both are white powders. Chloramine-T is used as a reagent in organic synthesis. It is commonly used as cyclizing agent in the synthesis of aziridine, oxadiazole, isoxazole and pyrazoles. It's inexpensive, has low toxicity and acts as a oxidizing agent. In addition, it also acts as a source of nitrogen anions and electrophilic cations. It may undergo degradation on long term exposure to atmosphere such that care must be taken during its storage.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove color from fabric or fiber or to disinfect after cleaning. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".

Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid.

Chlorine-releasing compounds, also known as chlorine base compounds, is jargon to describe certain chlorine-containing substances that are used as disinfectants and bleaches. They include the following chemicals: sodium hypochlorite, chloramine, halazone, and sodium dichloroisocyanurate. They are widely used to disinfect water and medical equipment, and surface areas as well as bleaching materials such as cloth. The presence of organic matter can make them less effective as disinfectants. They come as a liquid solution, or as a powder that is mixed with water before use.




















