In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two organyl groups. They have the general formula R−O−R′, where R and R′ represent organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of other enzymes, particularly lipases.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry.

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

Dimethyl terephthalate (DMT) is an organic compound with the formula C6H4(COOCH3)2. It is the diester formed from terephthalic acid and methanol. It is a white solid that melts to give a distillable colourless liquid.
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals.

Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is a commercially available reagent used in organic chemistry as a methylating agent and as a source of CH2 group. Its behavior is akin to the less convenient reagent diazomethane.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound, with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

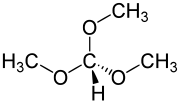
In organic chemistry, an ortho ester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula RC(OR′)3. Orthoesters may be considered as products of exhaustive alkylation of unstable orthocarboxylic acids and it is from these that the name 'ortho ester' is derived. An example is ethyl orthoacetate, CH3C(OCH2CH3)3, more correctly known as 1,1,1-triethoxyethane.

Triethyl orthoformate is an organic compound with the formula HC(OC2H5)3. This colorless volatile liquid, the ortho ester of formic acid, is commercially available. The industrial synthesis is from hydrogen cyanide and ethanol.

p-Toluenesulfonic acid (PTSA, pTSA, or pTsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white extremely hygroscopic solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C6H4SO2 group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O.

Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and as a co-solvent in lithium-ion batteries. Notably, dimethyl carbonate is a weak methylating agent, and is not considered as a carcinogen. Instead, dimethyl carbonate is often considered to be a green reagent, and it is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the United States.

Methyltrichlorosilane, also known as trichloromethylsilane, is a monomer and organosilicon compound with the formula CH3SiCl3. It is a colorless liquid with a sharp odor similar to that of hydrochloric acid. As methyltrichlorosilane is a reactive compound, it is mainly used a precursor for forming various cross-linked siloxane polymers.
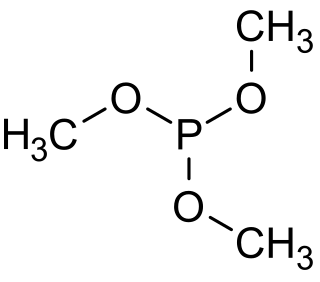
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.

3-Dimethylaminoacrolein is an organic compound with the formula Me2NC(H)=CHCHO. It is a pale yellow water-soluble liquid. The compound has a number of useful and unusual properties, e.g. it "causes a reversal of the hypnotic effect of morphine in mice" and has a "stimulating effect in humans".