 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.587 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
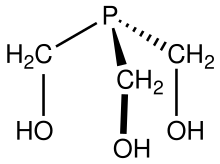
| C3H9O3P | |
| Molar mass | 124.076 g·mol−1 |
| Appearance | white solid |
| Density | 1.16 g/cm3 |
| Melting point | 51–53 °C (124–127 °F; 324–326 K) |
| Boiling point | decomposes |
| alcohols, dmf | |
| Hazards | |
| GHS labelling: [1] | |
   | |
| Danger | |
| H301, H315, H318, H335 | |
| P261, P264, P264+P265, P270, P271, P280, P301+P316, P302+P352, P304+P340, P305+P354+P338, P317, P319, P321, P330, P332+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tris(hydroxymethyl)phosphine is the organophosphorus compound with the formula P(CH2OH)3. It is a white solid. The compound is multifunctional, consisting of three alcohol functional groups and a tertiary phosphine. It is prepared by treating tetrakis(hydroxymethyl)phosphonium chloride with strong base: [2] [3]
- [P(CH2OH)4]Cl + NaOH → P(CH2OH)3 + H2O + H2C=O + NaCl
The compound can be prepared on a large scale using triethylamine as base and as solvent. [4]