
N-Acetylmannosamine is a hexosamine monosaccharide. It is a neutral, stable naturally occurring compound. N-Acetylmannosamine is also known as N-Acetyl-D-mannosamine monohydrate,, N-Acetyl-D-mannosamine which can be abbreviated to ManNAc or, less commonly, NAM). ManNAc is the first committed biological precursor of N-acetylneuraminic acid. Sialic acids are the negatively charged, terminal monosaccharides of carbohydrate chains that are attached to glycoproteins and glycolipids (glycans).
Epimerases and racemases are isomerase enzymes that catalyze the inversion of stereochemistry in biological molecules. Racemases catalyze the stereochemical inversion around the asymmetric carbon atom in a substrate having only one center of asymmetry. Epimerases catalyze the stereochemical inversion of the configuration about an asymmetric carbon atom in a substrate having more than one center of asymmetry, thus interconverting epimers.

Phosphatidylglycerol is a glycerophospholipid found in pulmonary surfactant and in the plasma membrane where it directly activates lipid-gated ion channels.
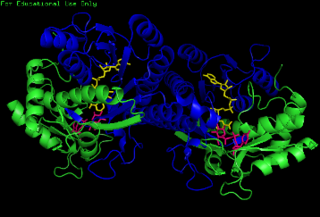
The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
In enzymology, an UDP-glucuronate 5'-epimerase is an enzyme that catalyzes the chemical reaction
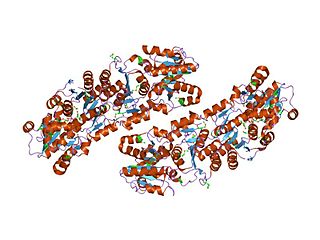
In enzymology, an UDP-N-acetylglucosamine 2-epimerase is an enzyme that catalyzes the chemical reaction
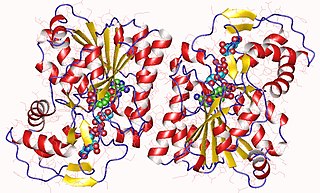
In enzymology, an UDP-N-acetylglucosamine 4-epimerase is an enzyme that catalyzes the chemical reaction
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.
In enzymology, a beta-galactoside alpha-2,6-sialyltransferase is an enzyme that catalyzes the chemical reaction

Bifunctional UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase is an enzyme that in humans is encoded by the GNE gene.
UDP-N-acetyl-2-amino-2-deoxyglucuronate dehydrogenase (EC 1.1.1.335, WlbA, WbpB) is an enzyme with systematic name UDP-N-acetyl-2-amino-2-deoxy-alpha-D-glucuronate:NAD+ 3-oxidoreductase. This enzyme catalyses the following chemical reaction:
UDP-4-amino-4,6-dideoxy-N-acetyl-alpha-D-glucosamine N-acetyltransferase is an enzyme with systematic name acetyl-CoA:UDP-4-amino-4,6-dideoxy-N-acetyl-alpha-D-glucosamine N-acetyltransferase. This enzyme catalyses the following chemical reaction
N,N'-diacetyllegionaminate synthase (EC 2.5.1.101, neuB (gene), legI (gene)) is an enzyme with systematic name phosphoenolpyruvate:2,4-diacetamido-2,4,6-trideoxy-alpha-D-mannopyranose 1-(2-carboxy-2-oxoethyl)transferase. This enzyme catalyses the following chemical reaction
UDP-4-amino-4,6-dideoxy-N-acetyl-alpha-D-glucosamine transaminase is an enzyme with systematic name UDP-4-amino-4,6-dideoxy-N-acetyl-alpha-D-glucosamine:2-oxoglutarate aminotransferase. This enzyme catalyses the following chemical reaction
CMP-N,N'-diacetyllegionaminic acid synthase is an enzyme with systematic name CTP:N,N'-diacetyllegionaminate cytidylyltransferase. This enzyme catalyses the following chemical reaction
UDP-N-acetylglucosamine 2-epimerase (hydrolysing) (EC 3.2.1.183, UDP-N-acetylglucosamine 2-epimerase, GNE (gene), siaA (gene), neuC (gene)) is an enzyme with systematic name UDP-N-acetyl-alpha-D-glucosamine hydrolase (2-epimerising). This enzyme catalyses the following chemical reaction

Epimerox is an experimental broad-spectrum antibiotic compound being developed by scientists at the Rockefeller University and Astex Pharmaceuticals. It is a small molecule inhibitor compound that blocks the activity of the enzyme UDP-N-acetylglucosamine 2-epimerase, an epimerase enzyme that is called 2-epimerase for short.
UDP-2,4-diacetamido-2,4,6-trideoxy-beta-L-altropyranose hydrolase (EC 3.6.1.57, PseG, UDP-6-deoxy-AltdiNAc hydrolase, Cj1312) is an enzyme with systematic name UDP-2,4-bis(acetamido)-2,4,6-trideoxy-beta-L-altropyranose hydrolase. This enzyme catalyses the following chemical reaction
UDP-N-acetylglucosamine 4,6-dehydratase (configuration-retaining) (EC 4.2.1.135, PglF) is an enzyme with systematic name UDP-N-acetyl-α-Dglucosamine hydro-lyase (configuration-retaining; UDP-2-acetamido-2,6-dideoxy-α-Dxylo-hex-4-ulose-forming). This enzyme catalyses the following chemical reaction
UDP-2,3-diacetamido-2,3-dideoxyglucuronic acid 2-epimerase is an enzyme with systematic name 2,3-diacetamido-2,3-dideoxy-alpha-D-glucuronate 2-epimerase. This enzyme catalyses the following chemical reaction







