
Heptane or n-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and iso-octane which is expressed as the percentage of iso-octane in heptane, and is listed on pumps for gasoline (petrol) dispensed globally.

1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula C
2H
4Br
2. Although trace amounts occur naturally in the ocean, where it is probably formed by algae and kelp, substantial amounts are produced industrially. It is a dense colorless liquid with a faint, sweet odor, detectable at 10 ppm. It is a widely used and sometimes-controversial fumigant. The combustion of 1,2-dibromoethane produces hydrogen bromide gas that is significantly corrosive.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.
Cyclohexene is a hydrocarbon with the formula (CH2)4C2H2. It is an example of a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an intermediate in the commercial synthesis of nylon.

Cyclohexanol is the organic compound with the formula HOCH(CH2)5. The molecule is related to cyclohexane by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colorless solid with a camphor-like odor, which, when very pure, melts near room temperature. Millions of tonnes are produced annually, mainly as a precursor to nylon.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C (255.7 °F). Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.

Bromoform is an organic compound with the chemical formula CHBr3. It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr. This volatile compound has an ether-like odor.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.

Glycidol is an organic compound with the formula HOCH2CHOCH2. The molecule contains both epoxide and alcohol functional groups. Being simple to make and bifunctional, it has a variety of industrial uses. The compound is a colorless, slightly viscous liquid that is slightly unstable and is not often encountered in pure form.

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.

Bromochloromethane or methylene bromochloride and Halon 1011 is a mixed halomethane. It is a heavy low-viscosity liquid with refractive index 1.4808.

Vinyl fluoride is an organic halide with the chemical formula C2H3F. It is a colorless gas with a faint ether-like odor. It is used as the monomeric precursor to the fluoropolymer polyvinylfluoride.

Chloromethyl methyl ether (CMME) is a compound with formula CH3OCH2Cl. A colorless liquid, it is a chloroalkyl ether. It is used as an alkylating agent. In organic synthesis, it is used for introducing the methoxymethyl ether (MOM) protecting group, and is thus often called MOM-Cl or MOM chloride. It also finds application as a chloromethylating agent in some variants of the Blanc chloromethylation.

Chloroacetaldehyde is an organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hemiacetal (ClCH2CH(OH))2O.
4-Nitrotoluene or para-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is a pale yellow solid. It is one of three isomers of nitrotoluene.

Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in synthesis of organic compounds, especially for vinylations.
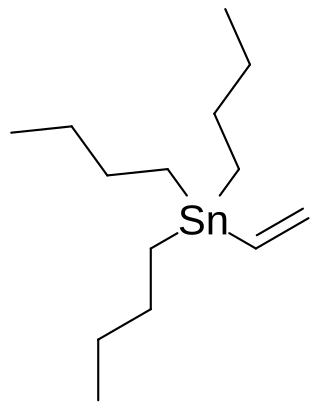
Vinyl tributyltin is an organotin compound with the formula Bu3SnCH=CH2 (Bu = butyl). It is a white, air-stable solid. It is used as a source of vinyl anion equivalent in Stille coupling reactions. As a source of vinyltin reagents, early work used vinyl trimethyltin, but trimethyltin compounds are avoided nowadays owing to their toxicity.




















