
Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:

β-Amylase is an enzyme with the systematic name 4-α-D-glucan maltohydrolase. It catalyses the following reaction:
The enzyme endoglycosylceramidase (EC 3.2.1.123) catalyzes the following chemical reaction:
In enzymology, a xyloglucan-specific exo-beta-1,4-glucanase (EC 3.2.1.155) is an enzyme that catalyzes the chemical reaction

In enzymology, a cyclomaltodextrin glucanotransferase is an enzyme that catalyzes the chemical reaction of cyclizing part of a 1,4-alpha-D-glucan molecule through the formation of a 1,4-alpha-D-glucosidic bond. They are bacterial enzymes belonging to the same family of the α-amylase specifically known as glycosyl-hydrolase family 13. This peculiar enzyme is capable of catalyzing more than one reaction with the most important being the synthesis of non-reducing cyclic dextrins known as cyclodextrins starting from starch, amylose, and other polysaccharides.

In molecular biology, a carbohydrate-binding module (CBM) is a protein domain found in carbohydrate-active enzymes. The majority of these domains have carbohydrate-binding activity. Some of these domains are found on cellulosomal scaffoldin proteins. CBMs were previously known as cellulose-binding domains. CBMs are classified into numerous families, based on amino acid sequence similarity. There are currently 64 families of CBM in the CAZy database.

In molecular biology, Glycoside hydrolase family 12 is a family of glycoside hydrolases.

In molecular biology, Glycoside hydrolase family 16 is a family of glycoside hydrolases.

In molecular biology, Glycoside hydrolase family 17 is a family of glycoside hydrolases. It folds into a TIM barrel.
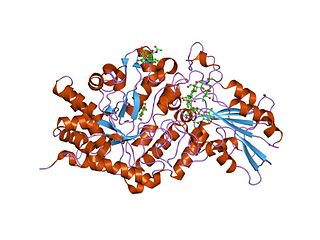
In molecular biology, glycoside hydrolase family 3 is a family of glycoside hydrolases. Glycoside hydrolases EC 3.2.1. are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycoside hydrolases, based on sequence similarity, has led to the definition of over 100 different families. This classification is available on the CAZy web site, and also discussed at CAZypedia, an online encyclopedia of carbohydrate active enzymes.
In molecular biology, glycoside hydrolase family 26 is a family of glycoside hydrolases.

Glucanases are enzymes that break down large polysaccharides via hydrolysis. The product of the hydrolysis reaction is called a glucan, a linear polysaccharide made of up to 1200 glucose monomers, held together with glycosidic bonds. Glucans are abundant in the endosperm cell walls of cereals such as barley, rye, sorghum, rice, and wheat. Glucanases are also referred to as lichenases, hydrolases, glycosidases, glycosyl hydrolases, and/or laminarinases. Many types of glucanases share similar amino acid sequences but vastly different substrates. Of the known endo-glucanases, 1,3-1,4-β-glucanase is considered the most active.
Endo-1,3(4)-β-glucanase -β-D-glucan 3(4)-glucanohydrolase) is an enzyme with systematic name 3(or 4)-β-D-glucan 3(4)-glucanohydrolase. It catalyses the following chemical reaction
Glucan endo-1,3-β-D-glucosidase is an enzyme with systematic name 3-β-D-glucan glucanohydrolase. This enzyme catalyses the following chemical reaction

Glucan 1,3-β-glucosidase is an enzyme with systematic name 3-β-D-glucan glucohydrolase. It catalyses the successive hydrolysis of β-D-glucose units from the non-reducing ends of (1→3)-β-D-glucans, releasing α-glucose.
Glucan endo-1,3-α-glucosidase is an enzyme with systematic name 3-α-D-glucan 3-glucanohydrolase. It catalyses endohydrolysis of (1→3)-α-D-glucosidic linkages in isolichenan, pseudonigeran and nigeran
Glucan endo-1,2-β-glucosidase is an enzyme with systematic name 2-β-D-glucan glucanohydrolase. It catalyses random hydrolysis of (1→2)-glucosidic linkages in (1→2)-β-D-glucans.
Lichenase is an enzyme with systematic name (1→3)-(1→4)-β-D-glucan 4-glucanohydrolase. It was named after its activity in on lichenin.
The enzyme glucan 1,4-β-glucosidase, also known as 4-β-D-glucan glucohydrolase, catalyses the hydrolysis of (1→4)-linkages in 1,4-β-D-glucans and related oligosaccharides, removing successive glucose units.
Glucan endo-1,6-β-glucosidase is an enzyme with systematic name 6-β-D-glucan glucanohydrolase. It catalyses random hydrolysis of (1→6)-linkages in (1→6)-β-D-glucans










