
A hypha is a long, branching filamentous structure of a fungus, oomycete, or actinobacterium. In most fungi, hyphae are the main mode of vegetative growth, and are collectively called a mycelium.

Neolecta is a genus of ascomycetous fungi that have fruiting bodies in the shape of unbranched to lobed bright yellowish, orangish to pale yellow-green colored, club-shaped, smooth, fleshy columns up to about 7 cm tall. The species share the English designation "Earth tongues" along with some better-known fungi with a similar general form, but in fact they are only distantly related.

Nematophagous fungi are carnivorous fungi specialized in trapping and digesting nematodes. Around 160 species are known. There exist both species that live inside the nematodes from the beginning and others that catch them, mostly with glue traps or in rings, some of which constrict on contact. Some species possess both types of traps. Another technique is to stun the nematodes using toxins, which is a method employed by Coprinus comatus, Stropharia rugosoannulata, and the family Pleurotaceae. The habit of feeding on nematodes has arisen many times among fungi, as is demonstrated by the fact that nematophagous species are found in all major fungal groups. Nematophagous fungi can be useful in controlling those nematodes that eat crops. Purpureocillium, for example, can be used as a bio-nematicide.

The Orbiliaceae are a family of saprobic sac fungi in the order Orbiliales. The family, first described by John Axel Nannfeldt in 1932, contains 288 species in 12 genera. Members of this family have a widespread distribution, but are more prevalent in temperate regions. Some species in the Orbiliaceae are carnivorous fungi, and have evolved a number of specialized mechanisms to trap nematodes.

The Zoopagomycotina are a subdivision of the fungal division Zygomycota sensu lato. It contains 5 families and 20 genera. Relationships among and within subphyla of Zygomycota are poorly understood, and their monophyly remains in question, so they are sometimes referred to by the informal name zygomycetes.

The Ancylistaceae are a family of fungi in the order Entomophthorales. The family currently contains 3 genera: Ancylistes, Macrobiotophthora, Conidiobolus.
Limnoperdon is a fungal genus in the monotypic family Limnoperdaceae. The genus is also monotypic, as it contains a single species, the aquatic fungus Limnoperdon incarnatum. The species, described as new to science in 1976, produces fruit bodies that lack specialized structures such as a stem, cap and gills common in mushrooms. Rather, the fruit bodies—described as aquatic or floating puffballs—are small balls of loosely interwoven hyphae. The balls float on the surface of the water above submerged twigs. Experimental observations on the development of the fruit body, based on the growth on the fungus in pure culture, suggest that a thin strand of mycelium tethers the ball above water while it matures. Fruit bodies start out as a tuft of hyphae, then become cup-shaped, and eventually enclose around a single chamber that contains reddish spores. Initially discovered in a marsh in the state of Washington, the fungus has since been collected in Japan, South Africa, and Canada.
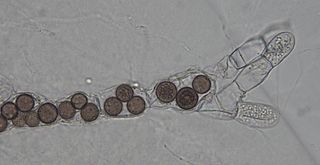
Rozella is a fungal genus of obligate endoparasites of a variety of hosts, including Oomycota, Chytridiomycota, and Blastocladiomycota. Rozella was circumscribed by French mycologist Marie Maxime Cornu in 1872. Considered one of the earliest diverging lineages of fungi, the widespread genus contains 27 species, with the most well studied being Rozella allomycis. Rozella is a member of a large clade of fungi referred to as the Cryptomycota/Rozellomycota. While some can be maintained in dual culture with the host, most have not been cultured, but they have been detected, using molecular techniques, in soil samples, and in freshwater and marine ecosystems. Zoospores have been observed, along with cysts, and the cells of some species are attached to diatoms.

Podostroma cornu-damae, also known the as poison fire coral, is a species of fungus in the family Hypocreaceae. The fruit bodies of the fungus are highly toxic, and have been responsible for several fatalities in Japan. The fungus contains several trichothecene mycotoxins.
Mycetophagites is an extinct fungal genus of mycoparasitic in the order Hypocreales. A monotypic genus, it contains the single species Mycetophagites atrebora.
Massarina carolinensis is a species of fungus in the Lophiostomataceae family. The species is found exclusively on the lower parts of the culms of the saltmarsh Juncus roemerianus on the Atlantic Coast of North Carolina.
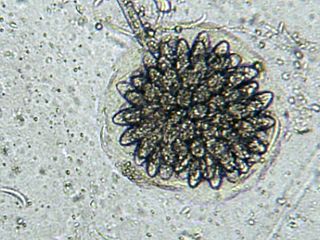
Rhopalomyces elegans is a common species of zygomycete fungus, and the type species of the genus Rhopalomyces. Widely distributed, it is found in soil, rotting plant material, and animal dung. It is a facultative parasite of nematode eggs.
Charles Drechsler was an American mycologist with 45 years of research with the United States Department of Agriculture. He spent considerable time working with cereal fungal diseases, and the genus Drechslera was named after him. Drechsler also worked extensively on oomycete fungi and their interactions with vegetable plants. Drechsler was recognized as a leading authority on helminthosporia, oomycetes, and other parasitic fungi.

Arthrobotrys oligospora was discovered in Europe in 1850 by Georg Fresenius. 'A. oligospora' is the model organism for interactions between fungi and nematodes. It is the most common nematode capturing fungus, and most widespread nematode trapping fungus in nature. It was the first species of fungi documented to actively capture nematodes.
Dactylellina haptotyla is a common soil-living fungus that develops structures to capture nematodes as nutrient source. In the presence of nematodes, spores can germinate into sticky knobs or non-constricting loops. The fungus traps nematodes with sticky knobs and non-constricting loops, then breakdown the cuticle, and penetrates the body of nematodes to obtain nutrients. For its predatory nature, Dactylellina haptotyla is also considered as nematode-trapping fungus or carnivorous fungus.
Physopella is a genus of fungal plant pathogen in the family Phakopsoraceae.

Keratinophyton durum is a keratinophilic fungus, that grows on keratin found in decomposing or shed animal hair and bird feathers. Various studies conducted in Canada, Japan, India, Spain, Poland, Ivory Coast and Iraq have isolated this fungus from decomposing animal hair and bird feathers using SDA and hair-bait technique. Presence of fungus in soil sediments and their ability to decompose hairs make them a potential human pathogen.
Acaulopage is a genus in the former Zygomycota that preys on amoeba.
Stylopage is a polytypic genus of predacious fungus in the order Zoopagales, within the subphylum Zoopagomycotina. All known species of Stylopage subsist on various species of amoebae or nematodes by trapping their prey, typically using an adhesive substance that coats their vegetative hyphae, and absorbing nutrients through the projection of a haustorium. 17 extant Stylopage species have been described thus far.

Chester Wilson Emmons was an American scientist, who researched fungi that cause diseases. He was the first mycologist at the National Institutes of Health (NIH), where for 31 years he served as head of its Medical Mycology Section.













