
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitute 1% of all described fungal species.

Sorbitol, less commonly known as glucitol, is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alcohol group (−CH2OH). Most sorbitol is made from potato starch, but it is also found in nature, for example in apples, pears, peaches, and prunes. It is converted to fructose by sorbitol-6-phosphate 2-dehydrogenase. Sorbitol is an isomer of mannitol, another sugar alcohol; the two differ only in the orientation of the hydroxyl group on carbon 2. While similar, the two sugar alcohols have very different sources in nature, melting points, and uses.

Xylitol is a chemical compound with the formula C
5H
12O
5, or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid. It is classified as a polyalcohol and a sugar alcohol, specifically an alditol. Of the common sugar alcohols, only sorbitol is more soluble in water.
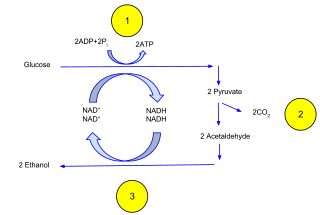
Ethanol fermentation, also called alcoholic fermentation, is a biological process which converts sugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as by-products. Because yeasts perform this conversion in the absence of oxygen, alcoholic fermentation is considered an anaerobic process. It also takes place in some species of fish where it provides energy when oxygen is scarce.
Cellulosic ethanol is ethanol produced from cellulose rather than from the plant's seeds or fruit. It can be produced from grasses, wood, algae, or other plants. It is generally discussed for use as a biofuel. The carbon dioxide that plants absorb as they grow offsets some of the carbon dioxide emitted when ethanol made from them is burned, so cellulosic ethanol fuel has the potential to have a lower carbon footprint than fossil fuels.

Xylulose is a ketopentose, a monosaccharide containing five carbon atoms, and including a ketone functional group. It has the chemical formula C5H10O5. In nature, it occurs in both the L- and D-enantiomers. 1-Deoxyxylulose is a precursor to terpenes via the DOXP pathway.

Clostridium acetobutylicum, ATCC 824, is a commercially valuable bacterium sometimes called the "Weizmann Organism", after Jewish Russian-born biochemist Chaim Weizmann. A senior lecturer at the University of Manchester, England, he used them in 1916 as a bio-chemical tool to produce at the same time, jointly, acetone, ethanol, and n-butanol from starch. The method has been described since as the ABE process,, yielding 3 parts of acetone, 6 of n-butanol, and 1 of ethanol. Acetone was used in the important wartime task of casting cordite. The alcohols were used to produce vehicle fuels and synthetic rubber.

In biochemistry, mixed acid fermentation is the metabolic process by which a six-carbon sugar is converted into a complex and variable mixture of acids. It is an anaerobic (non-oxygen-requiring) fermentation reaction that is common in bacteria. It is characteristic for members of the Enterobacteriaceae, a large family of Gram-negative bacteria that includes E. coli.

Lignocellulose refers to plant dry matter (biomass), so called lignocellulosic biomass. It is the most abundantly available raw material on the Earth for the production of biofuels. It is composed of two kinds of carbohydrate polymers, cellulose and hemicellulose, and an aromatic-rich polymer called lignin. Any biomass rich in cellulose, hemicelluloses, and lignin are commonly referred to as lignocellulosic biomass. Each component has a distinct chemical behavior. Being a composite of three very different components makes the processing of lignocellulose challenging. The evolved resistance to degradation or even separation is referred to as recalcitrance. Overcoming this recalcitrance to produce useful, high value products requires a combination of heat, chemicals, enzymes, and microorganisms. These carbohydrate-containing polymers contain different sugar monomers and they are covalently bound to lignin.

Fermentation is a type of redox metabolism carried out in the absence of oxygen. During fermentation, organic molecules are catabolized and donate electrons to other organic molecules. In the process, ATP and organic end products are formed.
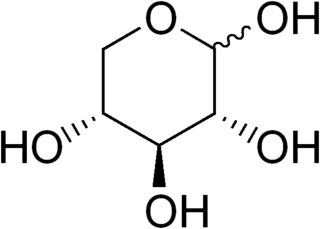
D-Xylose is a five-carbon aldose that can be catabolized or metabolized into useful products by a variety of organisms.

In enzymology, a D-xylulose reductase (EC 1.1.1.9) is an enzyme that is classified as an Oxidoreductase (EC 1) specifically acting on the CH-OH group of donors (EC 1.1.1) that uses NAD+ or NADP+ as an acceptor (EC 1.1.1.9). This enzyme participates in pentose and glucuronate interconversions; a set of metabolic pathways that involve converting pentose sugars and glucuronate into other compounds.

Sugars in wine are at the heart of what makes winemaking possible. During the process of fermentation, sugars from wine grapes are broken down and converted by yeast into alcohol (ethanol) and carbon dioxide. Grapes accumulate sugars as they grow on the grapevine through the translocation of sucrose molecules that are produced by photosynthesis from the leaves. During ripening the sucrose molecules are hydrolyzed (separated) by the enzyme invertase into glucose and fructose. By the time of harvest, between 15 and 25% of the grape will be composed of simple sugars. Both glucose and fructose are six-carbon sugars but three-, four-, five- and seven-carbon sugars are also present in the grape. Not all sugars are fermentable, with sugars like the five-carbon arabinose, rhamnose and xylose still being present in the wine after fermentation. Very high sugar content will effectively kill the yeast once a certain (high) alcohol content is reached. For these reasons, no wine is ever fermented completely "dry". Sugar's role in dictating the final alcohol content of the wine sometimes encourages winemakers to add sugar during winemaking in a process known as chaptalization solely in order to boost the alcohol content – chaptalization does not increase the sweetness of a wine.
Scheffersomyces stipitis is a species of yeast, belonging to the "CUG Clade" of ascomycetous yeasts. This is a group of fungi that substitute serine for leucine when the CUG codon is encountered. S. stipitis is distantly related to brewer's yeast, Saccharomyces cerevisiae, which uses the conventional codon system. Found, among other places, in the guts of passalid beetles, S. stipitis is capable of both aerobic and oxygen limited fermentation, and has the highest known natural ability of any yeast to directly ferment xylose, converting it to ethanol, a potentially economically valuable trait. Xylose is a hemicellulosic sugar found in all angiosperm plants. As such xylose constitutes the second most abundant carbohydrate moiety in nature. Xylose can be produced from wood or agricultural residues through auto- or acid hydrolysis. Ethanol production from such lignocellulosic residues does not compete with food production through the consumption of grain.

The role of yeast in winemaking is the most important element that distinguishes wine from fruit juice. In the absence of oxygen, yeast converts the sugars of the fruit into alcohol and carbon dioxide through the process of fermentation. The more sugars in the grapes, the higher the potential alcohol level of the wine if the yeast are allowed to carry out fermentation to dryness. Sometimes winemakers will stop fermentation early in order to leave some residual sugars and sweetness in the wine such as with dessert wines. This can be achieved by dropping fermentation temperatures to the point where the yeast are inactive, sterile filtering the wine to remove the yeast or fortification with brandy or neutral spirits to kill off the yeast cells. If fermentation is unintentionally stopped, such as when the yeasts become exhausted of available nutrients and the wine has not yet reached dryness, this is considered a stuck fermentation.
Ethanoligenens harbinense is a hydrogen producing, fermenting bacterium that shows potential for bioenergy-related applications, including biofuel production from waste streams..

In enzymology, a xylose isomerase is an enzyme that catalyzes the interconversion of D-xylose and D-xylulose. This enzyme belongs to the family of isomerases, specifically those intramolecular oxidoreductases interconverting aldoses and ketoses. The isomerase has now been observed in nearly a hundred species of bacteria. Xylose-isomerases are also commonly called glucose isomerase or fructose isomerases due to their ability to interconvert glucose and fructose. The systematic name of this enzyme class is α-D-xylopyranose aldose-ketose-isomerase. Other names in common use include D-xylose isomerase, D-xylose ketoisomerase, and D-xylose ketol-isomerase.
Aerobic fermentation or aerobic glycolysis is a metabolic process by which cells metabolize sugars via fermentation in the presence of oxygen and occurs through the repression of normal respiratory metabolism. Preference of aerobic fermentation over aerobic respiration is referred to as the Crabtree effect in yeast, and is part of the Warburg effect in tumor cells. While aerobic fermentation does not produce adenosine triphosphate (ATP) in high yield, it allows proliferating cells to convert nutrients such as glucose and glutamine more efficiently into biomass by avoiding unnecessary catabolic oxidation of such nutrients into carbon dioxide, preserving carbon-carbon bonds and promoting anabolism.
Propionispira raffinosivorans is a motile, obligate anaerobic, gram-negative bacteria. It was originally isolated from spoiled beer and believed to have some causative effect in beer spoilage. Since then, it has been taxonomically reclassified and proven to play a role in anaerobic beer spoilage, because of its production of acids, such as acetic and propionic acid, during fermentation
Kazachstania yasuniensis is a recently isolated yeast. This organism is part of the genus Kazachstania, which can be found in a large variety of habitats such as fermented foods, animals, wastewater, et cetera.













