Related Research Articles

A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, and enable mobility. Bones come in a variety of shapes and sizes and have complex internal and external structures. They are lightweight yet strong and hard and serve multiple functions.
A growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. Usually it is a secreted protein or a steroid hormone. Growth factors are important for regulating a variety of cellular processes.

A mammary gland is an exocrine gland in humans and other mammals that produces milk to feed young offspring. Mammals get their name from the Latin word mamma, "breast". The mammary glands are arranged in organs such as the breasts in primates, the udder in ruminants, and the dugs of other animals. Lactorrhea, the occasional production of milk by the glands, can occur in any mammal, but in most mammals, lactation, the production of enough milk for nursing, occurs only in phenotypic females who have gestated in recent months or years. It is directed by hormonal guidance from sex steroids. In a few mammalian species, male lactation can occur. With humans, male lactation can occur only under specific circumstances.
Osteoblasts are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon.

Parathyroid hormone-related protein (PTHrP) is a proteinaceous hormone and a member of the parathyroid hormone family secreted by mesenchymal stem cells. It is occasionally secreted by cancer cells. However, it also has normal functions in bone, teeth, vascular tissues and other tissues.

In cellular biology, paracrine signaling is a form of cell signaling, a type of cellular communication in which a cell produces a signal to induce changes in nearby cells, altering the behaviour of those cells. Signaling molecules known as paracrine factors diffuse over a relatively short distance, as opposed to cell signaling by endocrine factors, hormones which travel considerably longer distances via the circulatory system; juxtacrine interactions; and autocrine signaling. Cells that produce paracrine factors secrete them into the immediate extracellular environment. Factors then travel to nearby cells in which the gradient of factor received determines the outcome. However, the exact distance that paracrine factors can travel is not certain.

Platelet-derived growth factor (PDGF) is one among numerous growth factors that regulate cell growth and division. In particular, PDGF plays a significant role in blood vessel formation, the growth of blood vessels from already-existing blood vessel tissue, mitogenesis, i.e. proliferation, of mesenchymal cells such as fibroblasts, osteoblasts, tenocytes, vascular smooth muscle cells and mesenchymal stem cells as well as chemotaxis, the directed migration, of mesenchymal cells. Platelet-derived growth factor is a dimeric glycoprotein that can be composed of two A subunits (PDGF-AA), two B subunits (PDGF-BB), or one of each (PDGF-AB).

Osteoprotegerin (OPG), also known as osteoclastogenesis inhibitory factor (OCIF) or tumour necrosis factor receptor superfamily member 11B (TNFRSF11B), is a cytokine receptor of the tumour necrosis factor (TNF) receptor superfamily encoded by the TNFRSF11B gene.

An osteocyte, an oblate shaped type of bone cell with dendritic processes, is the most commonly found cell in mature bone. It can live as long as the organism itself. The adult human body has about 42 billion of them. Osteocytes do not divide and have an average half life of 25 years. They are derived from osteoprogenitor cells, some of which differentiate into active osteoblasts. Osteoblasts/osteocytes develop in mesenchyme.
Transforming growth factor is used to describe two classes of polypeptide growth factors, TGFα and TGFβ.

Fibroblast growth factor 2, also known as basic fibroblast growth factor (bFGF) and FGF-β, is a growth factor and signaling protein encoded by the FGF2 gene. It binds to and exerts effects via specific fibroblast growth factor receptor (FGFR) proteins, themselves a family of closely related molecules. Fibroblast growth factor protein was first purified in 1975; soon thereafter three variants were isolated: 'basic FGF' (FGF2); Heparin-binding growth factor-2; and Endothelial cell growth factor-2. Gene sequencing revealed that this group is the same FGF2 protein and is a member of a family of FGF proteins.

Bone resorption is resorption of bone tissue, that is, the process by which osteoclasts break down the tissue in bones and release the minerals, resulting in a transfer of calcium from bone tissue to the blood.

Sclerostin is a protein that in humans is encoded by the SOST gene. It is a secreted glycoprotein with a C-terminal cysteine knot-like (CTCK) domain and sequence similarity to the DAN family of bone morphogenetic protein (BMP) antagonists. Sclerostin is produced primarily by the osteocyte but is also expressed in other tissues, and has anti-anabolic effects on bone formation.
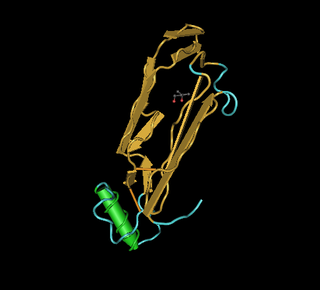
Bone morphogenetic protein 2 or BMP-2 belongs to the TGF-β superfamily of proteins.

Bone morphogenetic protein 4 is a protein that in humans is encoded by BMP4 gene. BMP4 is found on chromosome 14q22-q23.

Bone morphogenetic protein 6 is a protein that in humans is encoded by the BMP6 gene.

The bone morphogenetic protein receptor, type IA also known as BMPR1A is a protein which in humans is encoded by the BMPR1A gene. BMPR1A has also been designated as CD292.

Growth differentiation factor 2 (GDF2) also known as bone morphogenetic protein (BMP)-9 is a protein that in humans is encoded by the GDF2 gene. GDF2 belongs to the transforming growth factor beta superfamily.

Adipogenesis is the formation of adipocytes from stem cells. It involves 2 phases, determination, and terminal differentiation. Determination is mesenchymal stem cells committing to the adipocyte precursor cells, also known as preadipocytes which lose the potential to differentiate to other types of cells such as chondrocytes, myocytes, and osteoblasts. Terminal differentiation is that preadipocytes differentiate into mature adipocytes. Adipocytes can arise either from preadipocytes resident in adipose tissue, or from bone-marrow derived progenitor cells that migrate to adipose tissue.
The human skeletal system is a complex organ in constant equilibrium with the rest of the body. In addition to support and structure of the body, bone is the major reservoir for many minerals and compounds essential for maintaining a healthy pH balance. The deterioration of the body with age renders the elderly particularly susceptible to and affected by poor bone health. Illnesses like osteoporosis, characterized by weakening of the bone's structural matrix, increases the risk of hip-fractures and other life-changing secondary symptoms. In 2010, over 258,000 people aged 65 and older were admitted to the hospital for hip fractures. Incidence of hip fractures is expected to rise by 12% in America, with a projected 289,000 admissions in the year 2030. Other sources estimate up to 1.5 million Americans will have an osteoporotic-related fracture each year. The cost of treating these people is also enormous, in 1991 Medicare spent an estimated $2.9 billion for treatment and out-patient care of hip fractures, this number can only be expected to rise.
References
- 1 2 Mohan S, Baylink DJ (February 1991). "Bone growth factors". Clinical Orthopaedics and Related Research. 263 (263): 30–48. doi:10.1097/00003086-199102000-00004. PMID 1993386.
- 1 2 Baylink DJ, Finkelman RD, Mohan S (December 1993). "Growth factors to stimulate bone formation". Journal of Bone and Mineral Research. 8 Suppl 2: S565-72. doi:10.1002/jbmr.5650081326. PMID 8122528. S2CID 42984375.
- 1 2 3 4 Shim KS (March 2015). "Pubertal growth and epiphyseal fusion". Annals of Pediatric Endocrinology & Metabolism. 20 (1): 8–12. doi:10.6065/apem.2015.20.1.8. PMC 4397276 . PMID 25883921.
- ↑ Hausdorf J, Sievers B, Schmitt-Sody M, Jansson V, Maier M, Mayer-Wagner S (March 2011). "Stimulation of bone growth factor synthesis in human osteoblasts and fibroblasts after extracorporeal shock wave application". Archives of Orthopaedic and Trauma Surgery. 131 (3): 303–9. doi:10.1007/s00402-010-1166-4. PMID 20730589. S2CID 34915618.
- 1 2 3 4 Murray PG, Clayton PE (May 2013). "Endocrine control of growth". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 163C (2): 76–85. doi: 10.1002/ajmg.c.31357 . PMID 23613426. S2CID 3129856.
- ↑ Erlebacher A, Filvaroff EH, Ye JQ, Derynck R (July 1998). "Osteoblastic responses to TGF-beta during bone remodeling". Molecular Biology of the Cell. 9 (7): 1903–18. doi:10.1091/mbc.9.7.1903. PMC 25433 . PMID 9658179.
- 1 2 Ireland R (2020). Yeung CA (ed.). A Dictionary of Dentistry. doi:10.1093/acref/9780191828621.001.0001. ISBN 9780191828621.
- ↑ "Bone Growth Factors - Basic Science - Orthobullets". www.orthobullets.com. Retrieved 2021-05-05.
- ↑ Ying HZ, Chen Q, Zhang WY, Zhang HH, Ma Y, Zhang SZ, et al. (December 2017). "PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review)". Molecular Medicine Reports. 16 (6): 7879–7889. doi:10.3892/mmr.2017.7641. PMC 5779870 . PMID 28983598.
- ↑ Canalis E, McCarthy TL, Centrella M (September 1989). "Effects of platelet-derived growth factor on bone formation in vitro". Journal of Cellular Physiology. 140 (3): 530–7. doi:10.1002/jcp.1041400319. PMID 2777891. S2CID 43713808.
- ↑ Gimenez-Gallego G, Rodkey J, Bennett C, Rios-Candelore M, DiSalvo J, Thomas K (December 1985). "Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies". Science. 230 (4732): 1385–8. Bibcode:1985Sci...230.1385G. doi:10.1126/science.4071057. PMID 4071057.
- ↑ Yakar S, Werner H, Rosen CJ (July 2018). "Insulin-like growth factors: actions on the skeleton". Journal of Molecular Endocrinology. 61 (1): T115–T137. doi: 10.1530/JME-17-0298 . PMC 5966339 . PMID 29626053.
- ↑ Martin TJ (September 2005). "Osteoblast-derived PTHrP is a physiological regulator of bone formation". The Journal of Clinical Investigation. 115 (9): 2322–4. doi:10.1172/JCI26239. PMC 1193889 . PMID 16138187.
- ↑ Epigenetics and Cancer. Academic Press. 23 November 2010. pp. 62–. ISBN 978-0-12-380865-3.
- ↑ Helmuth Nyborg (1 January 1994). Hormones, Sex, and Society: The Science of Physicology. Greenwood Publishing Group. pp. 51–. ISBN 978-0-275-94608-1.
- ↑ Shaffer D, Kipp K (1 January 2013). Developmental Psychology: Childhood and Adolescence. Cengage Learning. pp. 191–. ISBN 978-1-111-83452-4.
- ↑ Lombardi G, Di Somma C, Rubino M, Faggiano A, Vuolo L, Guerra E, et al. (July 2011). "The roles of parathyroid hormone in bone remodeling: prospects for novel therapeutics". Journal of Endocrinological Investigation. 34 (7 Suppl): 18–22. PMID 21985975.
- ↑ Carter PH, Schipani E (March 2006). "The roles of parathyroid hormone and calcitonin in bone remodeling: prospects for novel therapeutics". Endocrine, Metabolic & Immune Disorders Drug Targets. 6 (1): 59–76. doi:10.2174/187153006776056666. PMID 16611165.
- ↑ "Osteoporosis Overview". NIH Osteoporosis and Related Bone Diseases National Resource Center.
- ↑ "Scientists discover new bone-forming growth factor that reverses osteoporosis in mice". ScienceDaily. Retrieved 2019-12-05.
- ↑ Yue R, Shen B, Morrison SJ (December 2016). "Clec11a/osteolectin is an osteogenic growth factor that promotes the maintenance of the adult skeleton". eLife. 5. doi:10.7554/eLife.18782. PMC 5158134 . PMID 27976999.
- 1 2 Al-Bluwi MT, Azam MQ, Sadat-Ali M (2016). "The effect of bone growth factor in the tendon to bone healing in anterior cruciate ligament reconstruction: An experimental study in rabbits". International Journal of Applied & Basic Medical Research. 6 (1): 23–7. doi:10.4103/2229-516X.174004. PMC 4765269 . PMID 26958518.
- 1 2 Anderson K, Seneviratne AM, Izawa K, Atkinson BL, Potter HG, Rodeo SA (2001–2011). "Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor". The American Journal of Sports Medicine. 29 (6): 689–98. doi:10.1177/03635465010290060301. PMID 11734478. S2CID 9946916.
- 1 2 Ma CB, Kawamura S, Deng XH, Ying L, Schneidkraut J, Hays P, Rodeo SA (April 2007). "Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin". The American Journal of Sports Medicine. 35 (4): 597–604. doi:10.1177/0363546506296312. PMID 17218656. S2CID 22750694.