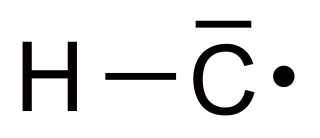In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.

The structural formula of a chemical compound is a graphic representation of the molecular structure, showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula.

In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.

Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. The term "unsaturated" means more hydrogen atoms may be added to the hydrocarbon to make it saturated. The configuration of an unsaturated carbons include straight chain, such as alkenes and alkynes, as well as branched chains and aromatic compounds.
The skeletal formula, or line-angle formula or shorthand formula, of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A skeletal formula shows the skeletal structure or skeleton of a molecule, which is composed of the skeletal atoms that make up the molecule. It is represented in two dimensions, as on a piece of paper. It employs certain conventions to represent carbon and hydrogen atoms, which are the most common in organic chemistry.
A substituent is one or a group of atoms that replaces atoms, thereby becoming a moiety in the resultant (new) molecule.
In organic chemistry, a methine group or methine bridge is a trivalent functional group =CH−, derived formally from methane. It consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen. The group is also called methyne or methene; its IUPAC systematic name is methylylidene or methanylylidene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.
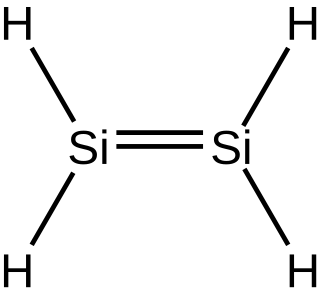
Silene, or silalkenes, are unsaturated hydrosilicons, which means that they consist only of hydrogen and silicon atoms and all bond, with the exception of one double bond, are either single or double bonds. By definition cycles are excluded, so that the silenes comprise homologous series of inorganic compounds with the general formula Si
nH
2n - 2k + 2, k > 0, where k is defined as the number of double bonds. There are no commercial sources.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
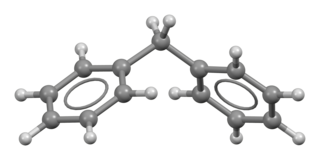
Diphenylmethane is an organic compound with the formula (C6H5)2CH2 (often abbreviated CH
2Ph
2). The compound consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. It is a white solid.
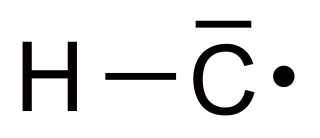
Methylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other functional groups for the hydrogen.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Methylene is an organic compound with the chemical formula CH
2. It is a colourless gas that fluoresces in the mid-infrared range, and only persists in dilution, or as an adduct.
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as CH2<, where the '<' denotes the two bonds. This can equally well be represented as −CH2−.
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula −CH2−; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of the molecule. It is the repeating unit in the skeleton of the unbranched alkanes.