
High-temperature superconductors are operatively defined as materials that behave as superconductors at temperatures above 77 K, the boiling point of liquid nitrogen, one of the simplest coolants in cryogenics. All materials currently known to superconduct at ordinary pressures become superconducting at temperatures far below ambient, and therefore require cooling. The majority of high-temperature superconductors are ceramic materials. On the other hand, Metallic superconductors usually work below −200 °C: they are then called low-temperature superconductors. Metallic superconductors are also ordinary superconductors, since they were discovered and used before the high-temperature ones.
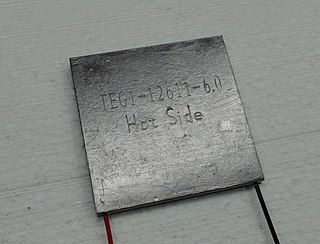
Thermoelectric materials show the thermoelectric effect in a strong or convenient form.

Covellite is a rare copper sulfide mineral with the formula CuS. This indigo blue mineral is commonly a secondary mineral in limited abundance and although it is not an important ore of copper itself, it is well known to mineral collectors.
Magnetic semiconductors are semiconductor materials that exhibit both ferromagnetism and useful semiconductor properties. If implemented in devices, these materials could provide a new type of control of conduction. Whereas traditional electronics are based on control of charge carriers, practical magnetic semiconductors would also allow control of quantum spin state. This would theoretically provide near-total spin polarization, which is an important property for spintronics applications, e.g. spin transistors.

Heusler compounds are magnetic intermetallics with face-centered cubic crystal structure and a composition of XYZ (half-Heuslers) or X2YZ (full-Heuslers), where X and Y are transition metals and Z is in the p-block. Many of these compounds exhibit properties relevant to spintronics, such as magnetoresistance, variations of the Hall effect, ferro-, antiferro-, and ferrimagnetism, half- and semimetallicity, semiconductivity with spin filter ability, superconductivity, and topological band structure. Their magnetism results from a double-exchange mechanism between neighboring magnetic ions. Manganese, which sits at the body centers of the cubic structure, was the magnetic ion in the first Heusler compound discovered. (See the Bethe–Slater curve for details of why this happens.)
In chemistry, hypomanganate, also called manganate(V) or tetraoxidomanganate(3−), is a trivalent anion composed of manganese and oxygen, with formula MnO3−
4.

Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a typical layered metal chalcogenide as it includes a group 16 anion (Se2−) and an electropositive element (Sn2+), and is arranged in a layered structure. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for applications including low-cost photovoltaics, and memory-switching devices.

Uranium nitride is any of a family of several ceramic materials: uranium mononitride (UN), uranium sesquinitride (U2N3) and uranium dinitride (UN2). The word nitride refers to the −3 oxidation state of the nitrogen bound to the uranium.

Lithium iron phosphate (LFP) is an inorganic compound with the formula LiFePO
4. It is a gray, red-grey, brown or black solid that is insoluble in water. The material has attracted attention as a component of lithium iron phosphate batteries, a type of Li-ion battery. This battery chemistry is targeted for use in power tools, electric vehicles, and solar energy installations. It is also used in OLPC XO education laptops.
Cuprate superconductors are a family of high-temperature superconducting materials made of layers of copper oxides (CuO2) alternating with layers of other metal oxides, which act as charge reservoirs. At ambient pressure, cuprate superconductors are the highest temperature superconductors known. However, the mechanism by which superconductivity occurs is still not understood.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research. A key disadvantage of organomanganese compounds is that they can be obtained directly from the metal only with difficulty.

The MAX phases are layered, hexagonal carbides and nitrides which have the general formula: Mn+1AXn, (MAX) where n = 1 to 4, and M is an early transition metal, A is an A-group (mostly IIIA and IVA, or groups 13 and 14) element and X is either carbon and/or nitrogen. The layered structure consists of edge-sharing, distorted XM6 octahedra interleaved by single planar layers of the A-group element.

Binary compounds of silicon are binary chemical compounds containing silicon and one other chemical element. Technically the term silicide is reserved for any compounds containing silicon bonded to a more electropositive element. Binary silicon compounds can be grouped into several classes. Saltlike silicides are formed with the electropositive s-block metals. Covalent silicides and silicon compounds occur with hydrogen and the elements in groups 10 to 17.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas on research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.
Antiperovskites is a type of crystal structure similar to the perovskite structure that is common in nature. The key difference is that the positions of the cation and anion constituents are reversed in the unit cell structure. In contrast to perovskite, antiperovskite compounds consist of two types of anions coordinated with one type of cation. Antiperovskite compounds are an important class of materials because they exhibit interesting and useful physical properties not found in perovskite materials.

Munirpallam Appadorai "Mas" Subramanian, often known also as M. A. Subramanian or Munirpallam Subramanian, is a solid-state chemist at Oregon State University in Corvallis, Oregon, and currently holds both the titles of Distinguished Professor and Milton Harris Chair of Materials Science in the Department of Chemistry. His work in solid-state chemistry on structure-property relationships of inorganic compounds has led to the discovery of several novel functional materials, many of which have found usage in various applications. Subramanian has authored 360 research publications and holds 60 patents. His publications have received more than 28,000 citations.
Sodium cobalt oxide, also called sodium cobaltate, is any of a range of compounds of sodium, cobalt, and oxygen with the general formula Na
xCoO
2 for 0 < x ≤ 1. The name is also used for hydrated forms of those compounds, Na
xCoO
2·yH
2O.
Nickel manganese oxides, or nickel manganates, are spinel structure compounds of Nickel, Manganese and Oxygen of the form: Ni(x)Mn(3-x)O(y)
Mixed anion compounds, heteroanionic materials or mixed anion materials are chemical compounds containing cations and more than one kind of anion. The compounds contain a single phase, rather than just a mixture.
The telluride oxides or oxytellurides are double salts that contain both telluride and oxide anions. They are in the class of mixed anion compounds.













