In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R−O−R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.

In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".

In organic chemistry, thioesters are organosulfur compounds with the molecular structure R−C(=O)−S−R’. They are analogous to carboxylate esters with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid with a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA. The R and R' represent organyl groups, or H in the case of R.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
Native Chemical Ligation (NCL) is an important extension of the chemical ligation concept for constructing a larger polypeptide chain by the covalent condensation of two or more unprotected peptides segments. Native chemical ligation is the most effective method for synthesizing native or modified proteins of typical size.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols. An exception is the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
The Willgerodt rearrangement or Willgerodt reaction is an organic reaction converting an aryl alkyl ketone, alkyne, or alkene to the corresponding amide by reaction with ammonium polysulfide, named after Conrad Willgerodt. The formation of the corresponding carboxylic acid is a side reaction resulting from hydrolysis of the amide. When the alkyl group is an aliphatic chain, multiple reactions take place with the amide group always ending up at the terminal end. The net effect is thus migration of the carbonyl group to the end of the chain and oxidation.

Thioacetic acid is an organosulfur compound with the molecular formula CH3C(O)SH. It is a thioic acid: the sulfur analogue of acetic acid, as implied by the thio- prefix. It is a yellow liquid with a strong thiol-like odor. It is used in organic synthesis for the introduction of thiol groups in molecules.
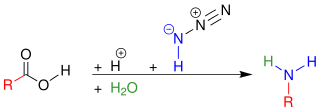
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. The intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products. The reaction is effective with carboxylic acids to give amines (above), and with ketones to give amides (below).

Diphenylphosphoryl azide (DPPA) is an organic compound. It is widely used as a reagent in the synthesis of other organic compounds.

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters where the carbon carries a higher oxidation state. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.

2,6-Pyridinedicarbothioic acid (PDTC) is an organosulfur compound that is produced by some bacteria. It functions as a, a low molecular weight compound that scavenges iron. Siderophores solubilize compounds by forming strong complexes. PDTC is secreted by the soil bacteria Pseudomonas stutzeri and Pseudomonas putida.

Sulfenamides (also spelled sulphenamides) are a class of organosulfur compounds characterized by the general formula RSNR'2, where R and R' are H, alkyl, or aryl. Sulfenamides have been used extensively in the vulcanization of rubber using sulfur. They are related to the oxidized compounds sulfinamides (RS(O)NR'2) and sulfonamides (RS(O)2NR'2).

Thiobenzoic acid is an organosulfur compound with molecular formula C6H5COSH. It is the parent of aryl thiocarboxylic acids. It is a pale yellow liquid that freezes just below room temperature.
















