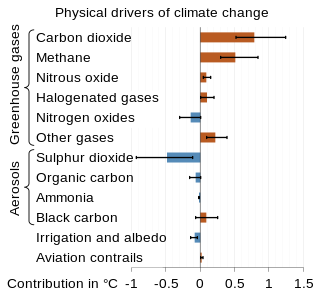
The scientific community has been investigating the causes of climate change for decades. After thousands of studies, the scientific consensus is that it is "unequivocal that human influence has warmed the atmosphere, ocean and land since pre-industrial times." This consensus is supported by around 200 scientific organizations worldwide. The scientific principle underlying current climate change is the greenhouse effect, which provides that greenhouse gases pass sunlight that heats the earth, but trap some of the resulting heat that radiates from the planet's surface. Large amounts of greenhouse gases such as carbon dioxide and methane have been released into the atmosphere through burning of fossil fuels since the industrial revolution. Indirect emissions from land use change, emissions of other greenhouse gases such as nitrous oxide, and increased concentrations of water vapor in the atmosphere, also contribute to climate change.

Global warming potential (GWP) is a measure of how much heat a greenhouse gas traps in the atmosphere over a specific time period, relative to carbon dioxide (CO2). It is expressed as a multiple of warming caused by the same mass of carbon dioxide (CO2). Therefore, by definition CO2 has a GWP of 1. For other gases it depends on how strongly the gas absorbs thermal radiation, how quickly the gas leaves the atmosphere, and the time frame considered.

An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. Though inert gases have a variety of applications, they are generally used to prevent unwanted chemical reactions with the oxygen (oxidation) and moisture (hydrolysis) in the air from degrading a sample. Generally, all noble gases except oganesson, nitrogen, and carbon dioxide are considered inert gases. The term inert gas is context-dependent because several of the inert gases, including nitrogen and carbon dioxide, can be made to react under certain conditions.

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.

A refrigerant is a working fluid used in cooling, heating or reverse cooling and heating of air conditioning systems and heat pumps where they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated because of their toxicity and flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change.
Eastman Chemical Company is an American company primarily involved in the chemical industry. Once a subsidiary of Kodak, today it is an independent global specialty materials company that produces a broad range of advanced materials, chemicals and fibers for everyday purposes. Founded in 1920 and based in Kingsport, Tennessee, the company operates 36 manufacturing sites worldwide and employs approximately 14,000 people.

Industrial wastewater treatment describes the processes used for treating wastewater that is produced by industries as an undesirable by-product. After treatment, the treated industrial wastewater may be reused or released to a sanitary sewer or to a surface water in the environment. Some industrial facilities generate wastewater that can be treated in sewage treatment plants. Most industrial processes, such as petroleum refineries, chemical and petrochemical plants have their own specialized facilities to treat their wastewaters so that the pollutant concentrations in the treated wastewater comply with the regulations regarding disposal of wastewaters into sewers or into rivers, lakes or oceans. This applies to industries that generate wastewater with high concentrations of organic matter, toxic pollutants or nutrients such as ammonia. Some industries install a pre-treatment system to remove some pollutants, and then discharge the partially treated wastewater to the municipal sewer system.

A fossil fuel power station is a thermal power station which burns a fossil fuel, such as coal, oil, or natural gas, to produce electricity. Fossil fuel power stations have machinery to convert the heat energy of combustion into mechanical energy, which then operates an electrical generator. The prime mover may be a steam turbine, a gas turbine or, in small plants, a reciprocating gas engine. All plants use the energy extracted from the expansion of a hot gas, either steam or combustion gases. Although different energy conversion methods exist, all thermal power station conversion methods have their efficiency limited by the Carnot efficiency and therefore produce waste heat.

Bioenergy is a type of renewable energy that is derived from plants and animal waste. The biomass that is used as input materials consists of recently living organisms, mainly plants. Thus, fossil fuels are not regarded as biomass under this definition. Types of biomass commonly used for bioenergy include wood, food crops such as corn, energy crops and waste from forests, yards, or farms.

1,1,1,2,3,3,3-Heptafluoropropane, also called heptafluoropropane, HFC-227ea, HFC-227 or FM-200, as well as apaflurane (INN), is a colourless, odourless gaseous halocarbon commonly used as a gaseous fire suppression agent.
Trifluoroiodomethane, also referred to as trifluoromethyl iodide is a halomethane with the formula CF3I. It is an experimental alternative to Halon 1301 (CBrF3) in unoccupied areas. It would be used as a gaseous fire suppression flooding agent for in-flight aircraft and electronic equipment fires.
Enhanced oil recovery, also called tertiary recovery, is the extraction of crude oil from an oil field that cannot be extracted otherwise. Whereas primary and secondary recovery techniques rely on the pressure differential between the surface and the underground well, enhanced oil recovery functions by altering the physical or chemical properties of the oil itself in order to make it easier to extract. When EOR is used, 30% to 60% or more of a reservoir's oil can be extracted, compared to 20% to 40% using only primary and secondary recovery.

Greenhouse gas (GHG) emissions from human activities intensify the greenhouse effect. This contributes to climate change. Carbon dioxide, from burning fossil fuels such as coal, oil, and natural gas, is the main cause of climate change. The largest annual emissions are from China followed by the United States. The United States has higher emissions per capita. The main producers fueling the emissions globally are large oil and gas companies. Emissions from human activities have increased atmospheric carbon dioxide by about 50% over pre-industrial levels. The growing levels of emissions have varied, but have been consistent among all greenhouse gases. Emissions in the 2010s averaged 56 billion tons a year, higher than any decade before. Total cumulative emissions from 1870 to 2022 were 703 GtC, of which 484±20 GtC from fossil fuels and industry, and 219±60 GtC from land use change. Land-use change, such as deforestation, caused about 31% of cumulative emissions over 1870–2022, coal 32%, oil 24%, and gas 10%.

The United States produced 5.2 billion metric tons of carbon dioxide equivalent greenhouse gas (GHG) emissions in 2020, the second largest in the world after greenhouse gas emissions by China and among the countries with the highest greenhouse gas emissions per person. In 2019 China is estimated to have emitted 27% of world GHG, followed by the United States with 11%, then India with 6.6%. In total the United States has emitted a quarter of world GHG, more than any other country. Annual emissions are over 15 tons per person and, amongst the top eight emitters, is the highest country by greenhouse gas emissions per person.

Methane is a chemical compound with the chemical formula CH4. It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure. In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Methane is an organic compound, and among the simplest of organic compounds. Methane is also a hydrocarbon.

Waste are unwanted or unusable materials. Waste is any substance discarded after primary use, or is worthless, defective and of no use. A by-product, by contrast is a joint product of relatively minor economic value. A waste product may become a by-product, joint product or resource through an invention that raises a waste product's value above zero.

A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work. The concept was originally applied solely to those materials capable of releasing chemical energy but has since also been applied to other sources of heat energy, such as nuclear energy.
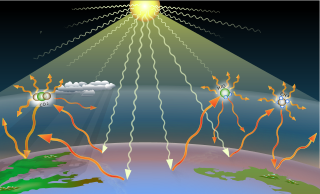
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F).
The United States Environmental Protection Agency (EPA) began regulating greenhouse gases (GHGs) under the Clean Air Act from mobile and stationary sources of air pollution for the first time on January 2, 2011. Standards for mobile sources have been established pursuant to Section 202 of the CAA, and GHGs from stationary sources are currently controlled under the authority of Part C of Title I of the Act. The basis for regulations was upheld in the United States Court of Appeals for the District of Columbia in June 2012.
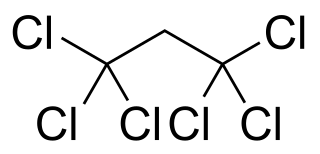
1,1,1,3,3,3-Hexachloropropane is a compound of chlorine, hydrogen, and carbon, with chemical formula C3Cl6H2, specifically Cl3C−CH2−CCl3. Its molecule can be described as that of propane with chlorine atoms substituted for the six hydrogen atoms on the extremal carbons.

















