 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,4-Dioxine [1] | |||
| Systematic IUPAC name 1,4-Dioxacyclohexa-2,5-diene | |||
| Other names 1,4-Dioxin Dioxin p-Dioxin 1,4-Dioxa[6]annulene | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.07 g/mol | ||
| Appearance | Colorless liquid | ||
| Boiling point | 75 °C (167 °F; 348 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | highly flammable | ||
| Related compounds | |||
Related compounds | 1,2-dioxin, dibenzodioxin | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
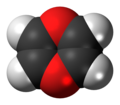
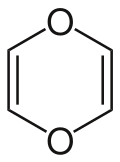
1,4-Dioxin (also referred as dioxin or p-dioxin) is a heterocyclic, organic, non-aromatic [2] compound with the chemical formula C4H4O2. There is an isomeric form of 1,4-dioxin, 1,2-dioxin (or o-dioxin). 1,2-Dioxin is very unstable due to its peroxide-like characteristics.
Contents
The term "dioxin" is most commonly used for a family of derivatives of 1,4-dioxin, known as polychlorinated dibenzodioxins (PCDDs).