In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.
In polymer chemistry, emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomers, and surfactants. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.
Acrylates are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.
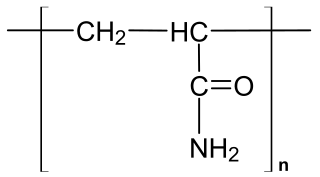
Polyacrylamide (abbreviated as PAM or pAAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly for water treatment and the paper and mineral industries.

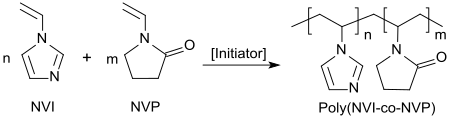
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material.

In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.

Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2Na)−]n and has broad applications in consumer products. This super-absorbent polymer (SAP) has the ability to absorb 100 to 1000 times its mass in water. Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. It is a polymer made up of chains of acrylate compounds. It contains sodium, which gives it the ability to absorb large amounts of water. When dissolved in water, it forms a thick and transparent solution due to the ionic interactions of the molecules. Sodium polyacrylate has many favorable mechanical properties. Some of these advantages include good mechanical stability, high heat resistance, and strong hydration. It has been used as an additive for food products including bread, juice, and ice cream.
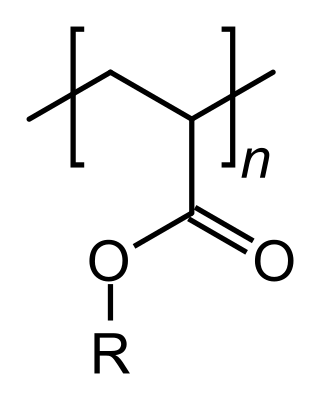
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.
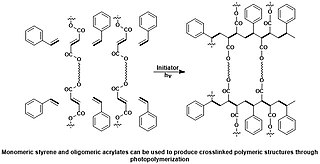
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing.

A superabsorbent polymer (SAP) (also called slush powder) is a water-absorbing hydrophilic homopolymers or copolymers that can absorb and retain extremely large amounts of a liquid relative to its own mass.

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.
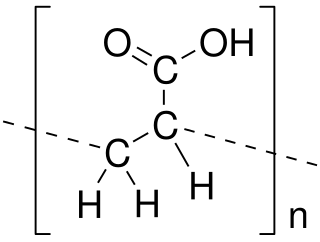
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid-base and water-attracting – are the bases of many applications.

N,N′-Methylenebisacrylamide (MBAm or MBAA, colloquially "bis") is the organic compound with the formula CH2[NHC(O)CH=CH2]2. A colorless solid, this compound is a crosslinking agent in polyacrylamides, e.g., as used for SDS-PAGE.

2-Acrylamido-2-methylpropane sulfonic acid (AMPS) was a Trademark name by The Lubrizol Corporation. It is a reactive, hydrophilic, sulfonic acid acrylic monomer used to alter the chemical properties of wide variety of anionic polymers. In the 1970s, the earliest patents using this monomer were filed for acrylic fiber manufacturing. Today, there are over several thousands patents and publications involving use of AMPS in many areas including water treatment, oil field, construction chemicals, hydrogels for medical applications, personal care products, emulsion coatings, adhesives, and rheology modifiers.
Living cationic polymerization is a living polymerization technique involving cationic propagating species. It enables the synthesis of very well defined polymers and of polymers with unusual architecture such as star polymers and block copolymers and living cationic polymerization is therefore as such of commercial and academic interest.
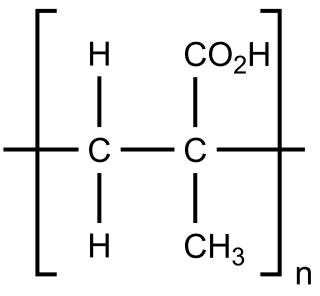
Poly(methacrylic acid) (PMAA) is a polymer made from methacrylic acid (preferred IUPAC name, 2-methylprop-2-enoic acid), which is a carboxylic acid. It is often available as its sodium salt, poly(methacrylic acid) sodium salt. The monomer is a viscous liquid with a pungent odour. The first polymeric form of methacrylic acid was described in 1880 by Engelhorn and Fittig. The use of high purity monomers is required for proper polymerization conditions and therefore it is necessary to remove any inhibitors by extraction (phenolic inhibitors) or via distillation. To prevent inhibition by dissolved oxygen, monomers should be carefully degassed prior to the start of the polymerization.

Vinylsulfonic acid is the organosulfur compound with the chemical formula CH2=CHSO3H. It is the simplest unsaturated sulfonic acid. The C=C double bond is a site of high reactivity. Polymerization gives polyvinylsulfonic acid, especially when used as a comonomer with functionalized vinyl and (meth)acrylic acid compounds. It is a colorless, water-soluble liquid, although commercial samples can appear yellow or even red.
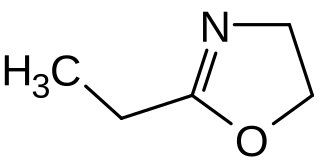
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s. This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.
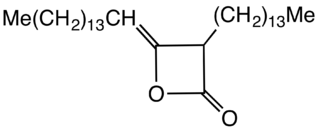
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position.
Dimethylaminoethyl acrylate or DMAEA is an unsaturated carboxylic acid ester having a tertiary amino group. It is a colorless to yellowish, water-miscible liquid with a pungent, amine-like odor. DMAEA is an important acrylic monomer that gives basic properties to copolymers.