
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.
Cuprates are a class of compounds that contain copper (Cu) atom(s) in an anion. They can be broadly categorized into two main types:

Phosphorine is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.

Bipyridines are a family of organic compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. Bipyridines, especially the 4,4' isomer, are mainly of significance in pesticides.

Terpyridine is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemistry.

1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refer to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.

Diethylenetriamine (abbreviated Dien or DETA) and also known as 2,2’-Iminodi(ethylamine)) is an organic compound with the formula HN(CH2CH2NH2)2. This colourless hygroscopic liquid is soluble in water and polar organic solvents, but not simple hydrocarbons. Diethylenetriamine is structural analogue of diethylene glycol. Its chemical properties resemble those for ethylene diamine, and it has similar uses. It is a weak base and its aqueous solution is alkaline. DETA is a byproduct of the production of ethylenediamine from ethylene dichloride.
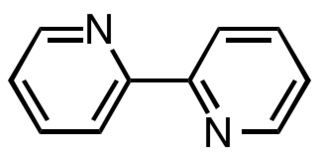
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence, which may have practical applications.

Tris(bipyridine)ruthenium(II) chloride is the chloride salt coordination complex with the formula [Ru(bpy)3]Cl2. This polypyridine complex is a red crystalline salt obtained as the hexahydrate, although all of the properties of interest are in the cation [Ru(bpy)3]2+, which has received much attention because of its distinctive optical properties. The chlorides can be replaced with other anions, such as PF6−.

Acetylenedicarboxylic acid or butynedioic acid is an organic compound with the formula H2C4O4 or HO−C(=O)−C≡C−C(=O)−OH. It is a crystalline solid that is soluble in diethyl ether.
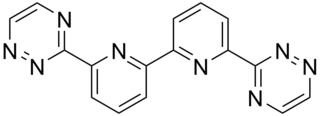
The bis-triazinyl bipyridines (BTBPs) are a class of chemical compounds which are tetradentate ligands similar in shape to quaterpyridine. The BTBPs are made by the reaction of hydrazine and a 1,2-diketone with 6,6'-dicyano-2,2'-bipyridine. The dicyanobipy can be made by reacting 2,2'-bipy with hydrogen peroxide in acetic acid, to form 2,2'-bipyridine-N,N-dioxide. The 2,2'-bipyridine-N,N-dioxide is then converted into the dicyano compound by treatment with potassium cyanide and benzoyl chloride in a mixture of water and THF.

2-Mercaptopyridine is an organosulfur compound with the formula HSC5H4N. This yellow crystalline solid is a derivative of pyridine. The compound and its derivatives serve primarily as acylating agents. A few of 2-mercaptopyridine's other uses include serving as a protecting group for amines and imides as well as forming a selective reducing agent. 2-Mercaptopyridine oxidizes to [[2,2′-dipyridyl disulfide]].

Chromium(VI) oxide peroxide is the name given to a collection of chromium coordination complexes. They have the formula CrO(O2)2L where L is a ligand. These species are dark blue and often labile. They all feature oxo ligand and two peroxo ligands, with the remaining coordination sites occupied by water, hydroxide, ether, or other Lewis bases.
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands.

2,2'-Dipyrromethene, often called just dipyrromethene or dipyrrin, is a chemical compound with formula C
9H
8N
2 whose skeleton can be described as two pyrrole rings C
5N connected by a methyne bridge =CH– through their nitrogen-adjacent (position-2) carbons; the remaining bonds being satisfied by hydrogen atoms. It is an unstable compound that is readily attacked by nucleophilic compounds above −40 °C.

cis-Dichlorobis(bipyridine)ruthenium(II) is the coordination complex with the formula RuCl2(bipy)2, where bipy is 2,2'-bipyridine. It is a dark green diamagnetic solid that is a precursor to many other complexes of ruthenium, mainly by substitution of the two chloride ligands. The compound has been crystallized as diverse hydrates.

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties.
Manganese(III) chloride is the hypothetical inorganic compound with the formula MnCl3.
Cobalt compounds are chemical compounds formed by cobalt with other elements.
















