In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (−OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.

Butylated hydroxytoluene (BHT), also known as dibutylhydroxytoluene, is a lipophilic organic compound, chemically a derivative of phenol, that is useful for its antioxidant properties. BHT is widely used to prevent free radical-mediated oxidation in fluids and other materials, and the regulations overseen by the U.S. F.D.A.—which considers BHT to be "generally recognized as safe"—allow small amounts to be added to foods. Despite this, and the earlier determination by the National Cancer Institute that BHT was noncarcinogenic in an animal model, societal concerns over its broad use have been expressed. BHT has also been postulated as an antiviral drug, but as of December 2022, use of BHT as a drug is not supported by the scientific literature and it has not been approved by any drug regulatory agency for use as an antiviral.

Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

2,4-Dimethyl-6-tert-butylphenol is the organic compound with the formula Me2(tert-Bu)C6H2OH (Me = methyl, tert-Bu = tertiary butyl). It is a colorless oil that is classified as an alkylated phenol.
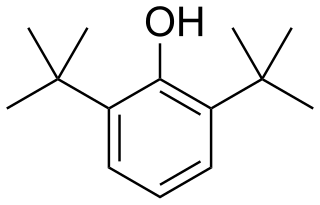
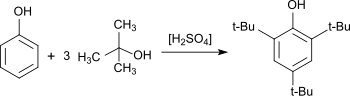
2,6-Di-tert-butylphenol is an organic compound with the structural formula 2,6-((CH3)3C)2C6H3OH. This colorless solid alkylated phenol and its derivatives are used industrially as UV stabilizers and antioxidants for hydrocarbon-based products ranging from petrochemicals to plastics. Illustrative of its usefulness, it prevents gumming in aviation fuels.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.
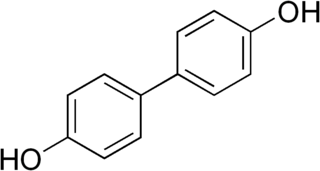
4,4′-Biphenol is an organic compound which is a phenolic derivative of biphenyl. It is a colorless solid.

2,6-Lutidine is a natural heterocyclic aromatic organic compound with the formula (CH3)2C5H3N. It is one of several dimethyl-substituted derivative of pyridine, all of which are referred to as lutidines. It is a colorless liquid with mildly basic properties and a pungent, noxious odor.

2,6-Di-tert-butylpyridine is an organic compound with the formula (Me3C)2C5H3N. This colourless, oily liquid is derived from pyridine by replacement of the two H atoms with tert-butyl groups. It is a hindered base. For example, it can be protonated, but it does not form an adduct with boron trifluoride.
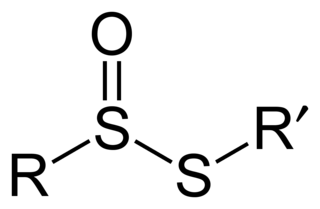
In organosulfur chemistry, thiosulfinate is a functional group consisting of the linkage R-S(O)-S-R. Thiolsulfinates are also named as alkanethiosulfinic acid esters.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Because the intracellular supply of protons is replenished, uncouplers actually stimulate cellular metabolism. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes.

A selenenic acid is an organoselenium compound and an oxoacid with the general formula RSeOH, where R ≠ H. It is the first member of the family of organoselenium oxoacids, which also include seleninic acids and selenonic acids, which are RSeO2H and RSeO3H, respectively. Selenenic acids derived from selenoenzymes are thought to be responsible for the antioxidant activity of these enzymes. This functional group is sometimes called SeO-selenoperoxol.

The White–Chen catalyst is an Iron-based coordination complex named after Professor M. Christina White and her graduate student Mark S. Chen. The catalyst is used along with hydrogen peroxide and acetic acid additive to oxidize aliphatic sp3 C-H bonds in organic synthesis. The catalyst is the first to allow for preparative and predictable aliphatic C–H oxidations over a broad range of organic substrates. Oxidations with the catalyst have proven to be remarkably predictable based on sterics, electronics, and stereoelectronics allowing for aliphatic C–H bonds to be thought of as a functional group in the streamlining of organic synthesis.
The Kharasch–Sosnovsky reaction is a method that involves using a copper or cobalt salt as a catalyst to oxidize olefins at the allylic position, subsequently condensing a peroxy ester or a peroxide resulting in the formation of allylic benzoates or alcohols via radical oxidation. This method is noteworthy for being the first allylic functionalization to utilize first-row transition metals and has found numerous applications in chemical and total synthesis. Chiral ligands can be used to render the reaction asymmetric, constructing chiral C–O bonds via C–H bond activation. This is notable as asymmetric addition to allylic groups tends to be difficult due to the transition state being highly symmetric. The reaction is named after Morris S. Kharasch and George Sosnovsky who first reported it in 1958. This method is noteworthy for being the first allylic functionalization to utilize first-row transition metals and has found numerous applications in chemical and total synthesis.

Transition metal isocyanide complexes are coordination compounds containing isocyanide ligands. Because isocyanides are relatively basic, but also good pi-acceptors, a wide range of complexes are known. Some isocyanide complexes are used in medical imaging.

2-tert-Butyl phenol is an organic compound with the formula (CH3)3CC6H4OH. It is one of three isomeric tert-butyl phenols. It is a colorless oil that dissolves in basic water. It can be prepared by acid-catalyzed alkylation of phenol with isobutene.

2,4,6-Tri-tert-butylpyrimidine is the organic compound with the formula HC(ButC)2N2CtBu where tBu = (CH3)3C. It is a substituted derivative of the heterocycle pyrimidine. Known also as TTBP, this compound is of interest as a base that is sufficiently bulky to not bind boron trifluoride but still able to bind protons. It is less expensive that the related bulky derivatives of pyridine such as 2,6-di-tert-butylpyridine, 2,4,6-tri-tert-butylpyridine, and 2,6-di-tert-butyl-4-methylpyridine.