A lubricant is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity.

Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH.

Heptane or n-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and iso-octane which is expressed as the percentage of iso-octane in heptane, and is listed on pumps for gasoline (petrol) dispensed globally.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

Synthetic oil is a lubricant consisting of chemical compounds that are artificially modified or synthesised. Synthetic lubricants can be manufactured using chemically modified petroleum components rather than whole crude oil, but can also be synthesized from other raw materials. The base material, however, is still overwhelmingly crude oil that is distilled and then modified physically and chemically. The actual synthesis process and composition of additives is generally a commercial trade secret and will vary among producers.
An antifreeze is an additive which lowers the freezing point of a water-based liquid. An antifreeze mixture is used to achieve freezing-point depression for cold environments. Common antifreezes also increase the boiling point of the liquid, allowing higher coolant temperature. However, all common antifreeze additives also have lower heat capacities than water, and do reduce water's ability to act as a coolant when added to it.
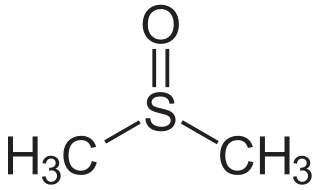
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula (CH3)2SO. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin.

Acetone is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.

In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: Si−O−Si. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H[OSiH2]nOH and [OSiH2]n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones [−R2Si−O−SiR2−]n, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
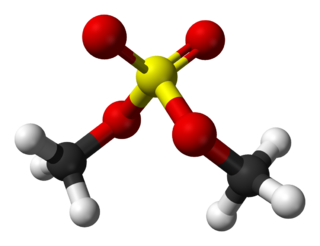
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis.
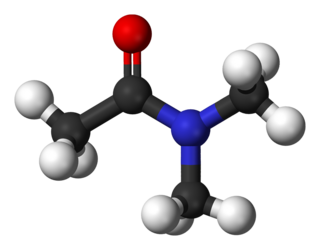
Dimethylacetamide (DMAc or DMA) is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water-miscible, high-boiling liquid is commonly used as a polar solvent in organic synthesis. DMA is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons.
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.
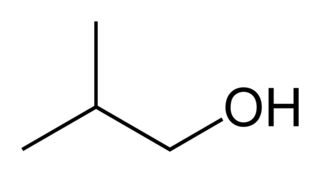
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and tert-butanol, all of which are important industrially.

Panthenol (also called pantothenol) is the alcohol analog of pantothenic acid (vitamin B5), and is thus a provitamin of B5. In organisms, it is quickly oxidized to pantothenic acid. It is a viscous transparent liquid at room temperature. Panthenol is used in pharmaceutical and cosmetic products as a moisturizer and to improve wound healing.
Glycol ethers are a class of chemical compounds consisting of alkyl ethers that are based on glycols such as ethylene glycol or propylene glycol. They are commonly used as solvents in paints and cleaners. They have good solvent properties while having higher boiling points than the lower-molecular-weight ethers and alcohols.

Hypromellose (INN), short for hydroxypropyl methylcellulose (HPMC), is a semisynthetic, inert, viscoelastic polymer used in eye drops, as well as an excipient and controlled-delivery component in oral medicaments, found in a variety of commercial products.
A crystallization adjutant is a material used to promote crystallization, normally in a context where a material does not crystallize naturally from a pure solution.

Racemic crystallography is a technique used in structural biology where crystals of a protein molecule are developed from an equimolar mixture of an L-protein molecule of natural chirality and its D-protein mirror image. L-protein molecules consist of 'left-handed' L-amino acids and the achiral amino acid glycine, whereas the mirror image D-protein molecules consist of 'right-handed' D-amino acids and glycine. Typically, both the L-protein and the D-protein are prepared by total chemical synthesis.

Protein crystallization is the process of formation of a regular array of individual protein molecules stabilized by crystal contacts. If the crystal is sufficiently ordered, it will diffract. Some proteins naturally form crystalline arrays, like aquaporin in the lens of the eye.















