
Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. The diphosphate group of ADP is attached to the 5’ carbon of the sugar backbone, while the adenine attaches to the 1’ carbon.

In biochemistry, a ribonucleotide is a nucleotide containing ribose as its pentose component. It is considered a molecular precursor of nucleic acids. Nucleotides are the basic building blocks of DNA and RNA. Ribonucleotides themselves are basic monomeric building blocks for RNA. Deoxyribonucleotides, formed by reducing ribonucleotides with the enzyme ribonucleotide reductase (RNR), are essential building blocks for DNA. There are several differences between DNA deoxyribonucleotides and RNA ribonucleotides. Successive nucleotides are linked together via phosphodiester bonds.

Nucleic acid metabolism is a collective term that refers to the variety of chemical reactions by which nucleic acids are either synthesized or degraded. Nucleic acids are polymers made up of a variety of monomers called nucleotides. Nucleotide synthesis is an anabolic mechanism generally involving the chemical reaction of phosphate, pentose sugar, and a nitrogenous base. Degradation of nucleic acids is a catabolic reaction and the resulting parts of the nucleotides or nucleobases can be salvaged to recreate new nucleotides. Both synthesis and degradation reactions require multiple enzymes to facilitate the event. Defects or deficiencies in these enzymes can lead to a variety of diseases.
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.

Guanosine monophosphate synthetase, also known as GMPS is an enzyme that converts xanthosine monophosphate to guanosine monophosphate.
In enzymology, an adenosine nucleosidase (EC 3.2.2.7) is an enzyme that catalyzes the chemical reaction
In enzymology, an adenosylhomocysteine nucleosidase (EC 3.2.2.9) is an enzyme that catalyzes the chemical reaction
In enzymology, an inosinate nucleosidase (EC 3.2.2.12) is an enzyme that catalyzes the chemical reaction
In enzymology, an inosine nucleosidase (EC 3.2.2.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a methylthioadenosine nucleosidase (EC 3.2.2.16) is an enzyme that catalyzes the chemical reaction
In enzymology, a NAD+ glycohydrolase (EC 3.2.2.5) is an enzyme that catalyzes the chemical reaction

In enzymology, a ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase (EC 3.2.2.6) is a bifunctional enzyme that catalyzes the chemical reaction
In enzymology, a N-methyl nucleosidase (EC 3.2.2.25) is an enzyme that catalyzes the chemical reaction
In enzymology, a NMN nucleosidase (EC 3.2.2.14) is an enzyme that catalyzes the chemical reaction
In enzymology, a purine nucleosidase (EC 3.2.2.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a pyrimidine-5'-nucleotide nucleosidase (EC 3.2.2.10) is an enzyme that catalyzes the chemical reaction
In enzymology, an uridine nucleosidase (EC 3.2.2.3) is an enzyme that catalyzes the chemical reaction
In enzymology, an adenosine-phosphate deaminase (EC 3.5.4.17) is an enzyme that catalyzes the chemical reaction
In enzymology, a S-methyl-5'-thioadenosine phosphorylase is an enzyme that catalyzes the chemical reaction
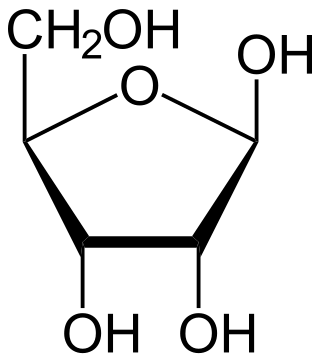
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, d-ribose, is a component of the ribonucleotides from which RNA is built, and so this compound is necessary for coding, decoding, regulation and expression of genes. It has a structural analog, deoxyribose, which is a similarly essential component of DNA. l-ribose is an unnatural sugar that was first prepared by Emil Fischer and Oscar Piloty in 1891. It was not until 1909 that Phoebus Levene and Walter Jacobs recognised that d-ribose was a natural product, the enantiomer of Fischer and Piloty's product, and an essential component of nucleic acids. Fischer chose the name "ribose" as it is a partial rearrangement of the name of another sugar, arabinose, of which ribose is an epimer at the 2' carbon; both names also relate to gum arabic, from which arabinose was first isolated and from which they prepared l-ribose.









