
Rusts are fungal plant pathogens of the order Pucciniales causing plant fungal diseases.

Brome mosaic virus (BMV) is a small, positive-stranded, icosahedral RNA plant virus belonging to the genus Bromovirus, family Bromoviridae, in the Alphavirus-like superfamily.
Barley yellow dwarf (BYD) is a plant disease caused by the barley yellow dwarf virus (BYDV), and is the most widely distributed viral disease of cereals. It affects the economically important crop species barley, oats, wheat, maize, triticale and rice.

Phyllody is the abnormal development of floral parts into leafy structures. It is generally caused by phytoplasma or virus infections, though it may also be because of environmental factors that result in an imbalance in plant hormones. Phyllody causes the affected plant to become partially or entirely sterile, as it is unable to produce normal flowers.

Eriophyidae is a family of more than 200 genera of mites, which live as plant parasites, commonly causing galls or other damage to the plant tissues and hence known as gall mites. About 3,600 species have been described, but this is probably less than 10% of the actual number existing in this poorly researched family. They are microscopic mites and are yellow to pinkish white to purplish in color. The mites are worm like, and have only two pairs of legs. Their primary method of population spread is by wind. They affect a wide range of plants, and several are major pest species causing substantial economic damage to crops. Some species, however, are used as biological agents to control weeds and invasive plant species.

Wheat leaf rust is a fungal disease that affects wheat, barley, rye stems, leaves and grains. In temperate zones it is destructive on winter wheat because the pathogen overwinters. Infections can lead up to 20% yield loss. The pathogen is a Puccinia rust fungus. It is the most prevalent of all the wheat rust diseases, occurring in most wheat-growing regions. It causes serious epidemics in North America, Mexico and South America and is a devastating seasonal disease in India. P. triticina is heteroecious, requiring two distinct hosts.

Cassava mosaic virus is the common name used to refer to any of eleven different species of plant pathogenic virus in the genus Begomovirus. African cassava mosaic virus (ACMV), East African cassava mosaic virus (EACMV), and South African cassava mosaic virus (SACMV) are distinct species of circular single-stranded DNA viruses which are transmitted by whiteflies and primarily infect cassava plants; these have thus far only been reported from Africa. Related species of viruses are found in India and neighbouring islands, though cassava is cultivated in Latin America as well as Southeast Asia. Nine species of cassava-infecting geminiviruses have been identified between Africa and India based on genomic sequencing and phylogenetic analysis. This number is likely to grow due to a high rate of natural transformation associated with CMV.
Barley stripe mosaic virus (BSMV), of genus Hordevirus, is an RNA viral plant pathogen whose main hosts are barley and wheat. The common symptoms for BSMV are yellow streaks or spots, mosaic, leaves and stunted growth. It is spread primarily through infected seed and can be spread through mechanical transfer of an infected and uninfected host. Plants infected with BSMV are more symptomatic in warmer temperatures. Resistant hosts and sterilization of equipment are the best ways to control the spread of the pathogen. BSMV has been known to reduce the yields of barley by up to 25%, but is not a major problem because of resistant varieties of barley.

Cymbidium mosaic virus (CymMV) is a plant pathogenic virus of the family Alphaflexiviridae.

Maize dwarf mosaic virus (MDMV) is a pathogenic plant virus of the family Potyviridae. Depending on the corn plant’s growth stage, the virus can have severe implications to the corn plant’s development which can also result in economic consequences to the producer of the crop.

Wheat streak mosaic virus (WSMV) is a plant pathogenic virus of the family Potyviridae that infects plants in the family Poaceae, especially wheat ; it is globally distributed and vectored by the wheat curl mite, particularly in regions where wheat is widely grown. First described in Nebraska in 1922, stunted growth and the eponymous “streaks” of yellowed, non-uniform discoloration are characteristic of WSMV infection. As it has been known to cause 100% crop mortality, WSMV is a subject of ongoing scientific research.
Soil-borne wheat mosaic virus is a rod-shaped plant pathogen that can cause severe stunting and mosaic in susceptible wheat, barley and rye cultivars. The disease has often been misdiagnosed as a nutritional problem, but this has actually allowed in part for the fortuitous visual selection by breeding programs of resistant genotypes. Soil-borne wheat mosaic virus is part of the genus Furovirus. Members of this genus are characterized by rigid rod-shaped particles and positive sense RNA genomes consisting of two molecules that are packaged into separate particles that code for either replication, mobility, structure or defense against the host. The virus is spread by a fungal-like protist, Polymyxa graminis, whose asexual secondary and sexual primary cycles help the virus spread. The disease produces secondary symptoms from the root cell infection. The disease is a serious contributor to loss in crop yield.

Aceria anthocoptes, also known as the russet mite, rust mite, thistle mite or the Canada thistle mite, is a species of mite that belongs to the family Eriophyidae. It was first described by Alfred Nalepa in 1892.
High plains disease is a viral disease afflicting wheat and maize. It is caused by the negative-sense ssRNA virus High Plains wheat mosaic emaravirus. Symptoms are similar to Wheat streak mosaic virus, with leaf veins showing yellow flecks and streaks, followed by leaf margin purpling in maize. Depending on the timing of infection, stunting and death occur. Plants can be doubly infected with high plains virus and wheat streak mosaic virus.
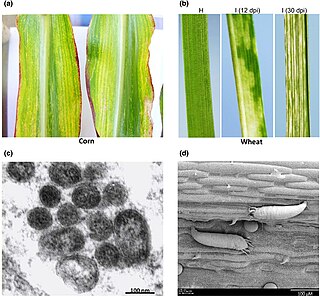
High Plains wheat mosaic emaravirus (WMoV), or High Plains virus (HPV) or Maize red stripe virus (MRSV/MRStV) is the causative agent of High plains disease of maize and wheat. It is spread by wheat curl mite, Aceria tosichella, which also transmits Wheat streak mosaic virus. The mite's ability to transmit a number of different viruses to cereal crops make it an economically important agricultural pest. In late June 2017 this virus was first detected in Canada, in Alberta. The Alberta samples were 99% similar to those in the USA. As Wheat streak mosaic virus is already present in Alberta, and coinfection with these two causes even more severe damage, this could cause much higher yield losses.

Aceria tosichella, commonly known as the wheat curl mite (WCM), is a global cereal pest and a vector for spreading and transmission of viruses like wheat streak mosaic virus (WSMV) and wheat mosaic virus (WMoV)
Maize lethal necrosis disease is a viral disease affecting maize (corn) predominantly in East Africa, Southeast Asia and South America, which was recognised in 2010. It is caused by simultaneous infection with two viruses, MCMoV and any of several Potyviridae.

Lolium rigidum is a species of annual grass. Common names by which it is known include annual ryegrass, a name also given to Italian ryegrass, rigid ryegrass, stiff darnel, Swiss ryegrass and Wimmera ryegrass. It is a native of southern Europe, northern Africa, the Middle East and the Indian subcontinent and is grown as a forage crop, particularly in Australia, where it is also a serious and economically damaging crop weed.
The cardamom mosaic virus (CdMV) is a mosaic virus that affects the production of green cardamom (E. cardamomum). It is a member of the genus Macluravirus (recognized under the family Potyviridae by ICTV in 1988), and is transmitted through aphids (P.caladii) and infected rhizomes, the former in a non-persistent manner.













