Related Research Articles

Mouthwash, mouth rinse, oral rinse, or mouth bath is a liquid which is held in the mouth passively or swilled around the mouth by contraction of the perioral muscles and/or movement of the head, and may be gargled, where the head is tilted back and the liquid bubbled at the back of the mouth.
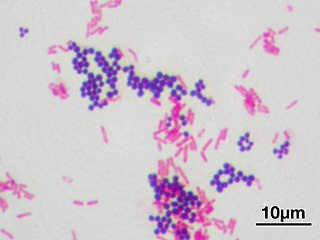
In microbiology and bacteriology, Gram stain, is a method of staining used to classify bacterial species into two large groups: gram-positive bacteria and gram-negative bacteria. The name comes from the Danish bacteriologist Hans Christian Gram, who developed the technique in 1884.

Phenol is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group bonded to a hydroxy group. Mildly acidic, it requires careful handling because it can cause chemical burns.

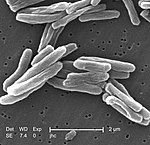
Mycobacterium is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis and leprosy in humans. The Greek prefix myco- means 'fungus', alluding to this genus' mold-like colony surfaces. Since this genus has cell walls with Gram-positive and Gram-negative features, acid-fast staining is used to emphasize their resistance to acids, compared to other cell types.

A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than sterilization, which is an extreme physical or chemical process that kills all types of life. Disinfectants are generally distinguished from other antimicrobial agents such as antibiotics, which destroy microorganisms within the body, and antiseptics, which destroy microorganisms on living tissue. Disinfectants are also different from biocides—the latter are intended to destroy all forms of life, not just microorganisms. Disinfectants work by destroying the cell wall of microbes or interfering with their metabolism. It is also a form of decontamination, and can be defined as the process whereby physical or chemical methods are used to reduce the amount of pathogenic microorganisms on a surface.
An antimicrobial is an agent that kills microorganisms or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. Agents that kill microbes are microbicides, while those that merely inhibit their growth are called bacteriostatic agents. The use of antimicrobial medicines to treat infection is known as antimicrobial chemotherapy, while the use of antimicrobial medicines to prevent infection is known as antimicrobial prophylaxis.
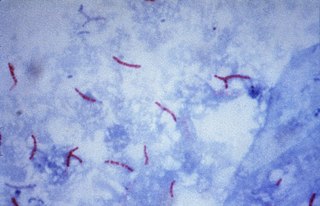
Ziehl–Neelsen staining is a type of acid-fast stain, first introduced by Paul Ehrlich. Ziehl–Neelsen staining is a bacteriological stain used to identify acid-fast organisms, mainly Mycobacteria. It is named for two German doctors who modified the stain: the bacteriologist Franz Ziehl (1859–1926) and the pathologist Friedrich Neelsen (1854–1898).

The auramine–rhodamine stain (AR), also known as the Truant auramine–rhodamine stain, is a histological technique used to visualize acid-fast bacilli using fluorescence microscopy, notably species in the Mycobacterium genus. Acid-fast organisms display a reddish-yellow fluorescence. Although the auramine–rhodamine stain is not as specific for acid-fast organisms as the Ziehl–Neelsen stain, it is more affordable and more sensitive, therefore it is often utilized as a screening tool.

Acid-fastness is a physical property of certain bacterial and eukaryotic cells, as well as some sub-cellular structures, specifically their resistance to decolorization by acids during laboratory staining procedures. Once stained as part of a sample, these organisms can resist the acid and/or ethanol-based decolorization procedures common in many staining protocols, hence the name acid-fast.
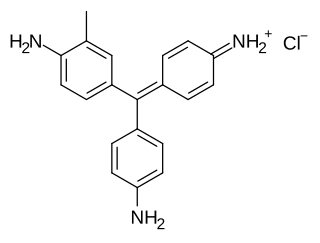
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl. There are other similar chemical formulations of products sold as fuchsine, and several dozen other synonyms of this molecule.

Nile blue is a stain used in biology and histology. It may be used with live or fixed cells, and imparts a blue colour to cell nuclei.

Mycobacterium smegmatis is an acid-fast bacterial species in the phylum Actinomycetota and the genus Mycobacterium. It is 3.0 to 5.0 µm long with a bacillus shape and can be stained by Ziehl–Neelsen method and the auramine-rhodamine fluorescent method. It was first reported in November 1884 by Lustgarten, who found a bacillus with the staining appearance of tubercle bacilli in syphilitic chancres. Subsequent to this, Alvarez and Tavel found organisms similar to that described by Lustgarten also in normal genital secretions (smegma). This organism was later named M. smegmatis.

Carbol fuchsin, carbol-fuchsin, carbolfuchsin, or Castellani's paint is a mixture of phenol and basic fuchsin that is used in bacterial staining procedures. It is commonly used in the staining of mycobacteria because it has an affinity for the mycolic acids found in their cell membranes.

Rhodamine B is a chemical compound and a dye. It is often used as a tracer dye within water to determine the rate and direction of flow and transport. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with fluorometers.

Auramine O is a diarylmethane dye used as a fluorescent stain. In its pure form, Auramine O appears as yellow needle crystals. It is insoluble in water and soluble in ethanol and DMSO.

Mycobacterium fortuitum is a nontuberculous species of the phylum Actinomycetota, belonging to the genus Mycobacterium.
In microbiology, the phenotypic testing of mycobacteria uses a number of methods. The most-commonly used phenotypic tests to identify and distinguish Mycobacterium strains and species from each other are described below.

Cleaning agents or hard-surface cleaners are substances used to remove dirt, including dust, stains, bad smells, and clutter on surfaces. Purposes of cleaning agents include health, beauty, removing offensive odor, and avoiding the spread of dirt and contaminants to oneself and others. Some cleaning agents can kill bacteria and clean at the same time. Others, called degreasers, contain organic solvents to help dissolve oils and fats.
The Kinyoun method or Kinyoun stain, developed by Joseph J. Kinyoun, is a procedure used to stain acid-fast species of the bacterial genus Mycobacterium. It is a variation of a method developed by Robert Koch in 1882. Certain species of bacteria have a waxy lipid called mycolic acid, in their cell walls which allow them to be stained with Acid-Fast better than a Gram-Stain. The unique ability of mycobacteria to resist decolorization by acid-alcohol is why they are termed acid-fast. It involves the application of a primary stain, a decolorizer (acid-alcohol), and a counterstain. Unlike the Ziehl–Neelsen stain, the Kinyoun method of staining does not require heating. In the Ziehl–Neelsen stain, heat acts as a physical mordant while phenol acts as the chemical mordant. Since the Kinyoun stain is a cold method, the concentration of carbol fuschin used is increased.
In microbiology, the term isolation refers to the separation of a strain from a natural, mixed population of living microbes, as present in the environment, for example in water or soil, or from living beings with skin flora, oral flora or gut flora, in order to identify the microbe(s) of interest. Historically, the laboratory techniques of isolation first developed in the field of bacteriology and parasitology, before those in virology during the 20th century.
References
- ↑ "Microbiology training log" . Retrieved 19 November 2012.
- ↑ "Comparison of the value of two different sputum staining for diagnosis of acid-fast bacilli" . Retrieved 19 November 2012.
- ↑ "Parasitology stains". Archived from the original on 10 April 2011. Retrieved 19 November 2012.