
Geminiviridae is a family of plant viruses that encode their genetic information on a circular genome of single-stranded (ss) DNA. There are 520 species in this family, assigned to 14 genera. Diseases associated with this family include: bright yellow mosaic, yellow mosaic, yellow mottle, leaf curling, stunting, streaks, reduced yields. They have single-stranded circular DNA genomes encoding genes that diverge in both directions from a virion strand origin of replication. According to the Baltimore classification they are considered class II viruses. It is the largest known family of single stranded DNA viruses.
Barley yellow dwarf (BYD) is a plant disease caused by the barley yellow dwarf virus (BYDV), and is the most widely distributed viral disease of cereals. It affects the economically important crop species barley, oats, wheat, maize, triticale and rice.

Aster yellows is a chronic, systemic plant disease caused by several bacteria called phytoplasma. The aster yellows phytoplasma (AYP) affects 300 species in 38 families of broad-leaf herbaceous plants, primarily in the aster family, as well as important cereal crops such as wheat and barley. Symptoms are variable and can include phyllody, virescence, chlorosis, stunting, and sterility of flowers. The aster leafhopper vector, Macrosteles quadrilineatus, moves the aster yellows phytoplasma from plant to plant. Its economic burden is primarily felt in the carrot crop industry, as well as the nursery industry. No cure is known for plants infected with aster yellows. Infected plants should be removed immediately to limit the continued spread of the phytoplasma to other susceptible plants. However, in agricultural settings such as carrot fields, some application of chemical insecticides has proven to minimize the rate of infection by killing the vector.

Curly top is a viral disease that affects many crops. This disease causes plants to become smaller in size, have shriveled petals and leaves, and are twisted and pulled out of shape. They are often caused by curtoviruses, members of the virus family Geminiviridae. This disease is important in western United States, such as California, Utah, Washington, and Idaho.

Pseudocercosporella capsellae is a plant pathogen infecting crucifers. P. capsellae is the causal pathogen of white leaf spot disease, which is an economically significant disease in global agriculture. P. capsellae has a significant affect on crop yields on agricultural products, such as canola seed and rapeseed. Researchers are working hard to find effective methods of controlling this plant pathogen, using cultural control, genetic resistance, and chemical control practices. Due to its rapidly changing genome, P. capsellae is a rapidly emerging plant pathogen that is beginning to spread globally and affect farmers around the world.
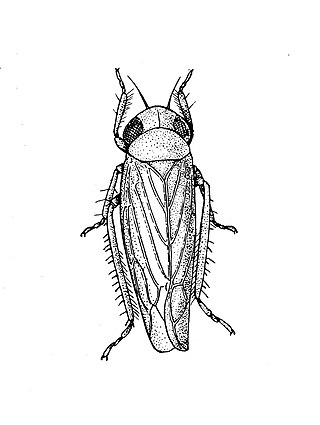
The beet leafhopper, also sometimes known as Neoaliturus tenellus, is a species of leafhopper which belongs to the family Cicadellidae in the order Hemiptera.
Diaporthe phaseolorum var. caulivora is a fungal plant pathogen which infects soybean, causing soybean stem canker.

Banana bunchy top virus (BBTV) is a plant pathogenic virus of the family Nanoviridae known for infecting banana plants and other crops. It is aphid transmitted.

Beet necrotic yellow vein virus (BNYVV) is a plant virus, transmitted by the plasmodiophorid Polymyxa betae. The BNYVV is a member of the genus Benyvirus and is responsible for rhizomania, a disease of sugar beet that causes proliferation of thin rootlets, and leads to a smaller tap root with reduced sugar content. Infected plants are less able to take up water, and wilting can be observed during the warm period of the year. If the infection spreads to the whole plant, vein yellowing, necrosis and yellow spots appear on the leaves, giving the virus its name.

Impatiens necrotic spot orthotospovirus(INSV) is a plant pathogenic virus of the order Bunyavirales. It was originally believed to be another strain of Tomato spotted wilt virus, but genetic investigations revealed them to be separate viruses. It is a negative-strand RNA virus which has a tripartite genome. It is largely spread by the insect vector of the western flower thrips. The virus infects more than 648 species of plants including important horticultural and agricultural species such as fuchsia, tomato, orchids, and lettuce (especially romaine). As the name implies, the main symptom on plants is necrotic spots that appear on the leaves. The INSV virus infects by injecting the RNA the virus contains into the cell which then starts using the cell resources to transcribe what the virus RNA states. Viral infection can often result in the death of the plant. The disease is mainly controlled by the elimination of the western flower thrip vector and by destroying any infected plant material.
Potato mop-top virus (PMTV) is a plant pathogenic virus transmitted through the vector Spongospora subterranea that affects potatoes. PMTV belongs to family of Virgaviridae, and the genus Pomovirus. The virus was first identified in 1966 by Calvert and Harrison in Britain, and is now reported in many other potato cultivating regions of the world including U.S.A., Canada, China, Pakistan, Japan, South American countries and many parts of Europe. Many disease management systems have been found to be ineffective against the virus, although a combination of sanitation and vector controls seems to work well.

Soybean mosaic virus (SMV) is a member of the plant virus genus Potyvirus. It infects mainly plants belonging to the family Fabaceae but has also been found infecting other economically important crops. SMV is the cause of soybean mosaic disease that occurs in all the soybean production areas of the world. Soybean is one of the most important sources of edible oil and proteins and pathogenic infections are responsible for annual yield losses of about $4 billion in the United States. Among these pathogens, SMV is the most important and prevalent viral pathogen in soybean production worldwide. It causes yield reductions of about 8% to 35%, but losses as high as 94% have been reported.
High plains disease is a viral disease afflicting wheat and maize. It is caused by the negative-sense ssRNA virus High Plains wheat mosaic emaravirus. Symptoms are similar to Wheat streak mosaic virus, with leaf veins showing yellow flecks and streaks, followed by leaf margin purpling in maize. Depending on the timing of infection, stunting and death occur. Plants can be doubly infected with high plains virus and wheat streak mosaic virus.
The Citrus stubborn disease is a plant disease affecting species in the genus Citrus. Spiroplasma citri, a Mollicute bacterium species, is the causative agent of the disease. It is present in the phloem of the affected plant. Originally discovered transmitted by several leafhoppers including Circulifer tenellus and Scaphytopius nitridus in citrus-growing regions of California, it is now spread by the same hoppers in Arizona and Circulifer haematoceps in the Mediterranean region.

Beet vascular necrosis and rot is a soft rot disease caused by the bacterium Pectobacterium carotovorum subsp. betavasculorum, which has also been known as Pectobacterium betavasculorum and Erwinia carotovora subsp. betavasculorum. It was classified in the genus Erwinia until genetic evidence suggested that it belongs to its own group; however, the name Erwinia is still in use. As such, the disease is sometimes called Erwinia rot today. It is a very destructive disease that has been reported across the United States as well as in Egypt. Symptoms include wilting and black streaks on the leaves and petioles. It is usually not fatal to the plant, but in severe cases the beets will become hollowed and unmarketable. The bacteria is a generalist species which rots beets and other plants by secreting digestive enzymes that break down the cell wall and parenchyma tissues. The bacteria thrive in warm and wet conditions, but cannot survive long in fallow soil. However, it is able to persist for long periods of time in the rhizosphere of weeds and non-host crops. While it is difficult to eradicate, there are cultural practices that can be used to control the spread of the disease, such as avoiding injury to the plants and reducing or eliminating application of nitrogen fertilizer.

Cherry X disease also known as Cherry Buckskin disease is caused by a plant pathogenic phytoplasma. Phytoplasmas are obligate parasites of plants and insects. They are specialized bacteria, characterized by their lack of a cell wall, often transmitted through insects, and are responsible for large losses in crops, fruit trees, and ornamentals. The phytoplasma causing Cherry X disease has a fairly limited host range mostly of stone fruit trees. Hosts of the pathogen include sweet cherry, sour cherry, choke cherry, peaches, nectarines, almonds, clover, and dandelion. Most commonly the pathogen is introduced into economical fruit orchards from wild choke cherry and herbaceous weed hosts. The pathogen is vectored by mountain and cherry leafhoppers. The mountain leafhopper vectors the pathogen from wild hosts to cherry orchards but does not feed on the other hosts. The cherry leafhopper feeds on cherry trees and can transmit the disease from cherry orchards to peach, nectarine, and other economic crops. Control of Cherry X disease is limited to controlling the spread, vectors, and weed hosts of the pathogen. Once the pathogen has infected a tree it is fatal and removal is necessary to stop it from becoming a reservoir for vectors.
Curtovirus is a genus of ssDNA viruses, in the family Geminiviridae. Dicotyledonous plants serve as natural hosts. Curtoviruses are transmitted by leafhoppers. There are three species in this genus. Diseases associated with this genus include: Curly top disease.
Papaya leaf curl virus(PaLCuV) is a DNA virus from the genus Begomovirus and the family Geminiviridae. PaLCuV causes severe disease in papaya (Carica papaya), but can sometimes infect other crops such as tobacco or tomato. It can be found in tropical and subtropical regions primarily in India, but closely related species have also been detected in countries such as China, Malaysia, Nigeria and South Korea. This virus is transmitted by an insect vector from the family Aleyrodidae and order Hemiptera, the whitefly Bemisia tabaci. PaLCuV has been responsible for several epidemics and causes severe economic losses. Because of the broad diversity of these viruses, their characterization and control remains difficult.
The cardamom mosaic virus (CdMV) is a mosaic virus that affects the production of green cardamom (E. cardamomum). It is a member of the genus Macluravirus (recognized under the family Potyviridae by ICTV in 1988), and is transmitted through aphids (P.caladii) and infected rhizomes, the former in a non-persistent manner.

Tomato brown rugose fruit virus (ToBRFV) is a plant virus in the genus Tobamovirus that was first described in 2015. It has spread rapidly since it was first noted in Jordan and Israel. The main hosts are tomato and peppers. The virus causes symptoms including mosaic and distortion of leaves and brown, wrinkly spots (rugose) on fruits. Outbreaks can be severe and leave fruit unmarketable.












