
The cochlea is the part of the inner ear involved in hearing. It is a spiral-shaped cavity in the bony labyrinth, in humans making 2.75 turns around its axis, the modiolus. A core component of the cochlea is the organ of Corti, the sensory organ of hearing, which is distributed along the partition separating the fluid chambers in the coiled tapered tube of the cochlea.
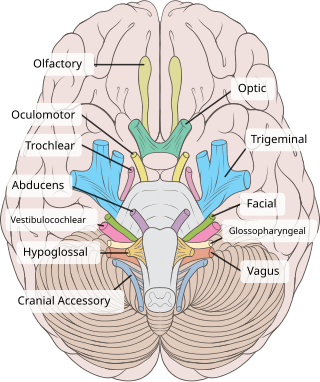
The vestibulocochlear nerve or auditory vestibular nerve, also known as the eighth cranial nerve, cranial nerve VIII, or simply CN VIII, is a cranial nerve that transmits sound and equilibrium (balance) information from the inner ear to the brain. Through olivocochlear fibers, it also transmits motor and modulatory information from the superior olivary complex in the brainstem to the cochlea.

In physiology, a stimulus is a change in a living thing's internal or external environment. This change can be detected by an organism or organ using sensitivity, and leads to a physiological reaction. Sensory receptors can receive stimuli from outside the body, as in touch receptors found in the skin or light receptors in the eye, as well as from inside the body, as in chemoreceptors and mechanoreceptors. When a stimulus is detected by a sensory receptor, it can elicit a reflex via stimulus transduction. An internal stimulus is often the first component of a homeostatic control system. External stimuli are capable of producing systemic responses throughout the body, as in the fight-or-flight response. In order for a stimulus to be detected with high probability, its level of strength must exceed the absolute threshold; if a signal does reach threshold, the information is transmitted to the central nervous system (CNS), where it is integrated and a decision on how to react is made. Although stimuli commonly cause the body to respond, it is the CNS that finally determines whether a signal causes a reaction or not.

The auditory system is the sensory system for the sense of hearing. It includes both the sensory organs and the auditory parts of the sensory system.

The lateral lemniscus is a tract of axons in the brainstem that carries information about sound from the cochlear nucleus to various brainstem nuclei and ultimately the contralateral inferior colliculus of the midbrain. Three distinct, primarily inhibitory, cellular groups are located interspersed within these fibers, and are thus named the nuclei of the lateral lemniscus.
In physiology, tonotopy is the spatial arrangement of where sounds of different frequency are processed in the brain. Tones close to each other in terms of frequency are represented in topologically neighbouring regions in the brain. Tonotopic maps are a particular case of topographic organization, similar to retinotopy in the visual system.

The medial geniculate nucleus (MGN) or medial geniculate body (MGB) is part of the auditory thalamus and represents the thalamic relay between the inferior colliculus (IC) and the auditory cortex (AC). It is made up of a number of sub-nuclei that are distinguished by their neuronal morphology and density, by their afferent and efferent connections, and by the coding properties of their neurons. It is thought that the MGN influences the direction and maintenance of attention.
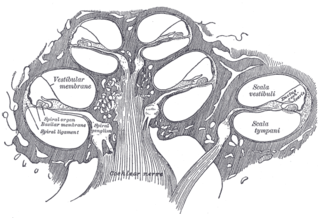
The cochlear nerve is one of two parts of the vestibulocochlear nerve, a cranial nerve present in amniotes, the other part being the vestibular nerve. The cochlear nerve carries auditory sensory information from the cochlea of the inner ear directly to the brain. The other portion of the vestibulocochlear nerve is the vestibular nerve, which carries spatial orientation information to the brain from the semicircular canals, also known as semicircular ducts.

The cochlear nucleus (CN) or cochlear nuclear complex comprises two cranial nerve nuclei in the human brainstem, the ventral cochlear nucleus (VCN) and the dorsal cochlear nucleus (DCN). The ventral cochlear nucleus is unlayered whereas the dorsal cochlear nucleus is layered. Auditory nerve fibers, fibers that travel through the auditory nerve carry information from the inner ear, the cochlea, on the same side of the head, to the nerve root in the ventral cochlear nucleus. At the nerve root the fibers branch to innervate the ventral cochlear nucleus and the deep layer of the dorsal cochlear nucleus. All acoustic information thus enters the brain through the cochlear nuclei, where the processing of acoustic information begins. The outputs from the cochlear nuclei are received in higher regions of the auditory brainstem.

The dorsal cochlear nucleus is a cortex-like structure on the dorso-lateral surface of the brainstem. Along with the ventral cochlear nucleus (VCN), it forms the cochlear nucleus (CN), where all auditory nerve fibers from the cochlea form their first synapses.

The superior olivary complex (SOC) or superior olive is a collection of brainstem nuclei that is located in pons, functions in multiple aspects of hearing and is an important component of the ascending and descending auditory pathways of the auditory system. The SOC is intimately related to the trapezoid body: most of the cell groups of the SOC are dorsal to this axon bundle while a number of cell groups are embedded in the trapezoid body. Overall, the SOC displays a significant interspecies variation, being largest in bats and rodents and smaller in primates.

The interaural time difference when concerning humans or animals, is the difference in arrival time of a sound between two ears. It is important in the localization of sounds, as it provides a cue to the direction or angle of the sound source from the head. If a signal arrives at the head from one side, the signal has further to travel to reach the far ear than the near ear. This pathlength difference results in a time difference between the sound's arrivals at the ears, which is detected and aids the process of identifying the direction of sound source.

In the ventral cochlear nucleus (VCN), auditory nerve fibers enter the brain via the nerve root in the VCN. The ventral cochlear nucleus is divided into the anterior ventral (anteroventral) cochlear nucleus (AVCN) and the posterior ventral (posteroventral) cochlear nucleus (PVCN). In the VCN, auditory nerve fibers bifurcate, the ascending branch innervates the AVCN and the descending branch innervates the PVCN and then continue to the dorsal cochlear nucleus. The orderly innervation by auditory nerve fibers gives the AVCN a tonotopic organization along the dorsoventral axis. Fibers that carry information from the apex of the cochlea that are tuned to low frequencies contact neurons in the ventral part of the AVCN; those that carry information from the base of the cochlea that are tuned to high frequencies contact neurons in the dorsal part of the AVCN. Several populations of neurons populate the AVCN. Bushy cells receive input from auditory nerve fibers through particularly large endings called end bulbs of Held. They contact stellate cells through more conventional boutons.

The calyx of Held is a particularly large excitatory synapse in the mammalian auditory nervous system, so named after Hans Held who first described it in his 1893 article Die centrale Gehörleitung because of its resemblance to the calyx of a flower. Globular bushy cells in the anteroventral cochlear nucleus (AVCN) send axons to the contralateral medial nucleus of the trapezoid body (MNTB), where they synapse via these calyces on MNTB principal cells. These principal cells then project to the ipsilateral lateral superior olive (LSO), where they inhibit postsynaptic neurons and provide a basis for interaural level detection (ILD), required for high frequency sound localization. This synapse has been described as the largest in the brain.
Coincidence detection is a neuronal process in which a neural circuit encodes information by detecting the occurrence of temporally close but spatially distributed input signals. Coincidence detectors influence neuronal information processing by reducing temporal jitter and spontaneous activity, allowing the creation of variable associations between separate neural events in memory. The study of coincidence detectors has been crucial in neuroscience with regards to understanding the formation of computational maps in the brain.
The olivocochlear system is a component of the auditory system involved with the descending control of the cochlea. Its nerve fibres, the olivocochlear bundle (OCB), form part of the vestibulocochlear nerve, and project from the superior olivary complex in the brainstem (pons) to the cochlea.
The neural encoding of sound is the representation of auditory sensation and perception in the nervous system. The complexities of contemporary neuroscience are continually redefined. Thus what is known of the auditory system has been continually changing. The encoding of sounds includes the transduction of sound waves into electrical impulses along auditory nerve fibers, and further processing in the brain.
Amblyaudia is a term coined by Dr. Deborah Moncrieff to characterize a specific pattern of performance from dichotic listening tests. Dichotic listening tests are widely used to assess individuals for binaural integration, a type of auditory processing skill. During the tests, individuals are asked to identify different words presented simultaneously to the two ears. Normal listeners can identify the words fairly well and show a small difference between the two ears with one ear slightly dominant over the other. For the majority of listeners, this small difference is referred to as a "right-ear advantage" because their right ear performs slightly better than their left ear. But some normal individuals produce a "left-ear advantage" during dichotic tests and others perform at equal levels in the two ears. Amblyaudia is diagnosed when the scores from the two ears are significantly different with the individual's dominant ear score much higher than the score in the non-dominant ear Researchers interested in understanding the neurophysiological underpinnings of amblyaudia consider it to be a brain based hearing disorder that may be inherited or that may result from auditory deprivation during critical periods of brain development. Individuals with amblyaudia have normal hearing sensitivity but have difficulty hearing in noisy environments like restaurants or classrooms. Even in quiet environments, individuals with amblyaudia may fail to understand what they are hearing, especially if the information is new or complicated. Amblyaudia can be conceptualized as the auditory analog of the better known central visual disorder amblyopia. The term “lazy ear” has been used to describe amblyaudia although it is currently not known whether it stems from deficits in the auditory periphery or from other parts of the auditory system in the brain, or both. A characteristic of amblyaudia is suppression of activity in the non-dominant auditory pathway by activity in the dominant pathway which may be genetically determined and which could also be exacerbated by conditions throughout early development.

Most owls are nocturnal or crepuscular birds of prey. Because they hunt at night, they must rely on non-visual senses. Experiments by Roger Payne have shown that owls are sensitive to the sounds made by their prey, not the heat or the smell. In fact, the sound cues are both necessary and sufficient for localization of mice from a distant location where they are perched. For this to work, the owls must be able to accurately localize both the azimuth and the elevation of the sound source.
Bushy cells are two types of second order neuron found in the anterior part of the ventral cochlear nucleus, the AVCN. They can be globular or spherical giving outputs to different parts of the superior olivary complex.













