
The visual cortex of the brain is the area of the cerebral cortex that processes visual information. It is located in the occipital lobe. Sensory input originating from the eyes travels through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. The area of the visual cortex that receives the sensory input from the lateral geniculate nucleus is the primary visual cortex, also known as visual area 1 (V1), Brodmann area 17, or the striate cortex. The extrastriate areas consist of visual areas 2, 3, 4, and 5.

The visual system is the physiological basis of visual perception. The system detects, transduces and interprets information concerning light within the visible range to construct an image and build a mental model of the surrounding environment. The visual system is associated with the eye and functionally divided into the optical system and the neural system.

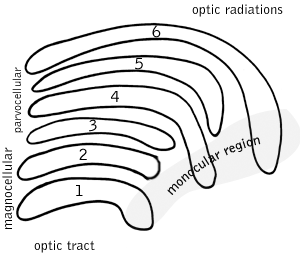
In neuroanatomy, the lateral geniculate nucleus is a structure in the thalamus and a key component of the mammalian visual pathway. It is a small, ovoid, ventral projection of the thalamus where the thalamus connects with the optic nerve. There are two LGNs, one on the left and another on the right side of the thalamus. In humans, both LGNs have six layers of neurons alternating with optic fibers.

The occipital lobe is one of the four major lobes of the cerebral cortex in the brain of mammals. The name derives from its position at the back of the head, from the Latin ob, 'behind', and caput, 'head'.
The receptive field, or sensory space, is a delimited medium where some physiological stimuli can evoke a sensory neuronal response in specific organisms.

Motion perception is the process of inferring the speed and direction of elements in a scene based on visual, vestibular and proprioceptive inputs. Although this process appears straightforward to most observers, it has proven to be a difficult problem from a computational perspective, and difficult to explain in terms of neural processing.
Stereopsis is the component of depth perception retrieved by means of binocular disparity through binocular vision. It is not the only contributor to depth perception, but it is a major one. Binocular vision occurs because each eye receives a different image due to their slightly different positions in one's head. These positional differences are referred to as "horizontal disparities" or, more generally, "binocular disparities". Disparities are processed in the visual cortex of the brain to yield depth perception. While binocular disparities are naturally present when viewing a real three-dimensional scene with two eyes, they can also be simulated by artificially presenting two different images separately to each eye using a method called stereoscopy. The perception of depth in such cases is also referred to as "stereoscopic depth".
In neuroscience, a koniocellular cell is a neuron with a small cell body that is located in the koniocellular layer of the lateral geniculate nucleus (LGN) of the thalamus of primates, including humans.
In neuroscience, neuronal tuning refers to the hypothesized property of brain cells by which they selectively represent a particular type of sensory, association, motor, or cognitive information. Some neuronal responses have been hypothesized to be optimally tuned to specific patterns through experience. Neuronal tuning can be strong and sharp, as observed in primary visual cortex, or weak and broad, as observed in neural ensembles. Single neurons are hypothesized to be simultaneously tuned to several modalities, such as visual, auditory, and olfactory. Neurons hypothesized to be tuned to different signals are often hypothesized to integrate information from the different sources. In computational models called neural networks, such integration is the major principle of operation. The best examples of neuronal tuning can be seen in the visual, auditory, olfactory, somatosensory, and memory systems, although due to the small number of stimuli tested the generality of neuronal tuning claims is still an open question.
The two-streams hypothesis is a model of the neural processing of vision as well as hearing. The hypothesis, given its initial characterisation in a paper by David Milner and Melvyn A. Goodale in 1992, argues that humans possess two distinct visual systems. Recently there seems to be evidence of two distinct auditory systems as well. As visual information exits the occipital lobe, and as sound leaves the phonological network, it follows two main pathways, or "streams". The ventral stream leads to the temporal lobe, which is involved with object and visual identification and recognition. The dorsal stream leads to the parietal lobe, which is involved with processing the object's spatial location relative to the viewer and with speech repetition.

The inferior temporal gyrus is one of three gyri of the temporal lobe and is located below the middle temporal gyrus, connected behind with the inferior occipital gyrus; it also extends around the infero-lateral border on to the inferior surface of the temporal lobe, where it is limited by the inferior sulcus. This region is one of the higher levels of the ventral stream of visual processing, associated with the representation of objects, places, faces, and colors. It may also be involved in face perception, and in the recognition of numbers and words.

The efficient coding hypothesis was proposed by Horace Barlow in 1961 as a theoretical model of sensory coding in the brain. Within the brain, neurons communicate with one another by sending electrical impulses referred to as action potentials or spikes. One goal of sensory neuroscience is to decipher the meaning of these spikes in order to understand how the brain represents and processes information about the outside world. Barlow hypothesized that the spikes in the sensory system formed a neural code for efficiently representing sensory information. By efficient it is understood that the code minimized the number of spikes needed to transmit a given signal. This is somewhat analogous to transmitting information across the internet, where different file formats can be used to transmit a given image. Different file formats require different number of bits for representing the same image at given distortion level, and some are better suited for representing certain classes of images than others. According to this model, the brain is thought to use a code which is suited for representing visual and audio information representative of an organism's natural environment.
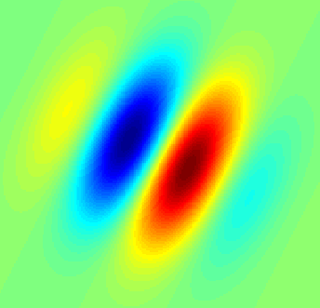
A simple cell in the primary visual cortex is a cell that responds primarily to oriented edges and gratings. These cells were discovered by Torsten Wiesel and David Hubel in the late 1950s.
Complex cells can be found in the primary visual cortex (V1), the secondary visual cortex (V2), and Brodmann area 19 (V3).

The colour centre is a region in the brain primarily responsible for visual perception and cortical processing of colour signals received by the eye, which ultimately results in colour vision. The colour centre in humans is thought to be located in the ventral occipital lobe as part of the visual system, in addition to other areas responsible for recognizing and processing specific visual stimuli, such as faces, words, and objects. Many functional magnetic resonance imaging (fMRI) studies in both humans and macaque monkeys have shown colour stimuli to activate multiple areas in the brain, including the fusiform gyrus and the lingual gyrus. These areas, as well as others identified as having a role in colour vision processing, are collectively labelled visual area 4 (V4). The exact mechanisms, location, and function of V4 are still being investigated.

A hypercomplex cell is a type of visual processing neuron in the mammalian cerebral cortex. Initially discovered by David Hubel and Torsten Wiesel in 1965, hypercomplex cells are defined by the property of end-stopping, which is a decrease in firing strength with increasingly larger stimuli. The sensitivity to stimulus length is accompanied by selectivity for the specific orientation, motion, and direction of stimuli. For example, a hypercomplex cell may only respond to a line at 45˚ that travels upward. Elongating the line would result in a proportionately weaker response. Ultimately, hypercomplex cells can provide a means for the brain to visually perceive corners and curves in the environment by identifying the ends of a given stimulus.
Globs are millimeter-sized color modules found beyond the visual area V2 in the brain's color processing ventral pathway. They are scattered throughout the posterior inferior temporal cortex in an area called the V4 complex. They are clustered by color preference, and organized as color columns. They are the first part of the brain in which color is processed in terms of the full range of hues found in color space.
Feature detection is a process by which the nervous system sorts or filters complex natural stimuli in order to extract behaviorally relevant cues that have a high probability of being associated with important objects or organisms in their environment, as opposed to irrelevant background or noise.

A parasol cell, sometimes called an M cell or M ganglion cell, is one type of retinal ganglion cell (RGC) located in the ganglion cell layer of the retina. These cells project to magnocellular cells in the lateral geniculate nucleus (LGN) as part of the magnocellular pathway in the visual system. They have large cell bodies as well as extensive branching dendrite networks and as such have large receptive fields. Relative to other RGCs, they have fast conduction velocities. While they do show clear center-surround antagonism, they receive no information about color. Parasol ganglion cells contribute information about the motion and depth of objects to the visual system.
Surround suppression is where the relative firing rate of a neuron may under certain conditions decrease when a particular stimulus is enlarged. It has been observed in electrophysiology studies of the brain and has been noted in many sensory neurons, most notably in the early visual system. Surround suppression is defined as a reduction in the activity of a neuron in response to a stimulus outside its classical receptive field.












