
Dothideomycetes is the largest and most diverse class of ascomycete fungi. It comprises 11 orders 90 families, 1,300 genera and over 19,000 known species. Wijayawardene et al. in 2020 added more orders to the class.
Leptosphaeria maculans is a fungal pathogen of the phylum Ascomycota that is the causal agent of blackleg disease on Brassica crops. Its genome has been sequenced, and L. maculans is a well-studied model phytopathogenic fungus. Symptoms of blackleg generally include basal stem cankers, small grey lesions on leaves, and root rot. The major yield loss is due to stem canker. The fungus is dispersed by the wind as ascospores or rain splash in the case of the conidia. L. maculans grows best in wet conditions and a temperature range of 5–20 degrees Celsius. Rotation of crops, removal of stubble, application of fungicide, and crop resistance are all used to manage blackleg. The fungus is an important pathogen of Brassica napus (canola) crops.
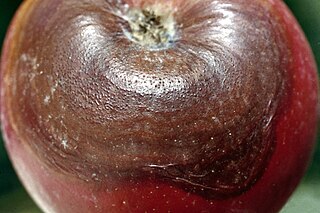
Botryosphaeria obtusa is a plant pathogen that causes frogeye leaf spot, black rot and cankers on many plant species. On the leaf it is referred to as frogeye leaf spot; this phase typically affects tree and shrubs. In fruit such as the apple, cranberry and quince, it is referred to as black rot, and in twigs and trunks it causes cankers.
Leptosphaeria coniothyrium is a plant pathogen. It can be found around the world.

Lasiodiplodia theobromae is a plant pathogen with a very wide host range. It causes rotting and dieback in most species it infects. It is a common post harvest fungus disease of citrus known as stem-end rot. It is a cause of bot canker of grapevine. It also infects Biancaea sappan, a species of flowering tree also known as Sappanwood.

Botryosphaeria ribis is a fungal plant pathogen that infects many trees causing cankers, dieback and death.

The Pleosporales is the largest order in the fungal class Dothideomycetes. By a 2008 estimate, it contained 23 families, 332 genera and more than 4700 species. The majority of species are saprobes on decaying plant material in fresh water, marine, or terrestrial environments, but several species are also associated with living plants as parasites, epiphytes or endophytes. The best studied species cause plant diseases on important agricultural crops e.g. Cochliobolus heterostrophus, causing southern corn leaf blight on maize, Phaeosphaeria nodorum causing glume blotch on wheat and Leptosphaeria maculans causing a stem canker on cabbage crops (Brassica). Some species of Pleosporales occur on animal dung, and a small number occur as lichens and rock-inhabiting fungi.

The Botryosphaeriaceae are a family of sac fungi (Ascomycetes), which is the type representative of the order Botryosphaeriales. According to a 2008 estimate, the family contains 26 genera and over 1500 species. Members of this order include notable plant pathogens.

Neofabraea is a genus of fungi in the family Dermateaceae. The genus contains 12 species.
Neoscytalidium is a genus of fungi in the Botryosphaeriaceae family.
Bot canker of oak is a disease on stems, branches and twigs of oak trees in Europe and North America. The casual agent of Bot canker of oak is the fungus Botryosphaeria corticola. Bot canker of oak causes lesions and cankers on a wide range of oaks in Europe and most recently live oaks in North America. Some infections were formerly attributed to Botryosphaeria stevensii, but most likely represent infections by Botryosphaeria corticola. Botryosphaeria corticola is distinguishable from Botryosphaeria stevensii via ITS rDNA sequencing.
Phaeobotryosphaeria is a genus of fungi in the family Botryosphaeriaceae. There were originally 10 species.

The Magnaporthaceae are a family of fungi in the order Magnaporthales. It was circumscribed by Paul F. Cannon in 1994 for a group of grass-associated fungi centered on Magnaporthe (Nakataea). Magnaporthaceae have a harpophora-like asexual morphology and are often associated with roots of grasses or cereals.
Grapevine trunk diseases (GTD) are the most destructive diseases of vineyards worldwide. Fungicides with the potential to control GTD have been banned in Europe and there are no highly effective treatments available. Action to develop new strategies to fight these diseases are needed.
Fusicoccum ramosum is an endophytic fungus that might be a canker pathogen, specifically for Adansonia gibbosa (baobab). It was isolated from said trees, as well as surrounding ones, in the Kimberley.

Neoscytalidium dimidiatum was first described in 1933 as Hendersonula toruloidea from diseased orchard trees in Egypt. Decades later, it was determined to be a causative agent of human dermatomycosis-like infections and foot infections predominantly in tropical areas; however the fungus is considered to be widespread. A newer name, Scytalidium dimidiatum, was applied to a synanamorph of Nattrassia mangiferae, otherwise known as Neofusicoccum mangiferae. Substantial confusion has arisen in the literature on this fungus resulting from the use of multiple different names including Torula dimidiata, Fusicoccum dimidiatum, Scytalidium dimidiatum, and Hendersonula toruloidea. Additionally, Scytalidium lignicola and Scytalidium lignicolum are often considered earlier names of N. dimidiatum.
The Pyriculariaceae are a family of ascomycete fungi in the order Magnaporthales. It was introduced by S. Klaubauf, M.H. Lebrun & P.W. Crous in 2014.
Neofusicoccum arbuti is a fungus species in the genus Neofusicoccum. It was first described by D.F. Farr & M. Elliott, and given its current name by Crous, Slippers & A.J.L. Phillips in 2006. Neofusicoccum arbuti is included in the genus Neofusicoccum and the family Botryosphaeriaceae. This species is known as madrone canker. N. arbuti is a potentially lethal canker disease of Pacific madrone, Arbutus menziesii.

Coniothyriaceae is a family of ascomycetous marine based fungi within the order of Pleosporales in the subclass Pleosporomycetidae and within the class Dothideomycetes. They are pathogenic or they can be saprobic on dead branches. They are generally a anamorphic species.
The Sphaeropsis are a genus of fungi, within the family of Botryosphaeriaceae and within the order of Botryosphaeriales, within the class Dothideomycetes. They are plant pathogens.











