
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables.
Carotenoids are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, archaea, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, canaries, flamingos, salmon, lobster, shrimp, and daffodils. Over 1,100 identified carotenoids can be further categorized into two classes – xanthophylls and carotenes.

Xanthophylls are yellow pigments that occur widely in nature and form one of two major divisions of the carotenoid group; the other division is formed by the carotenes. The name is from Greek: xanthos (ξανθός), meaning "yellow", and phyllon (φύλλον), meaning "leaf"), due to their formation of the yellow band seen in early chromatography of leaf pigments.

Astaxanthin is a keto-carotenoid within a group of chemical compounds known as terpenes. Astaxanthin is a metabolite of zeaxanthin and canthaxanthin, containing both hydroxyl and ketone functional groups. It is a lipid-soluble pigment with red coloring properties, which result from the extended chain of conjugated double bonds at the center of the compound.
Retinal is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
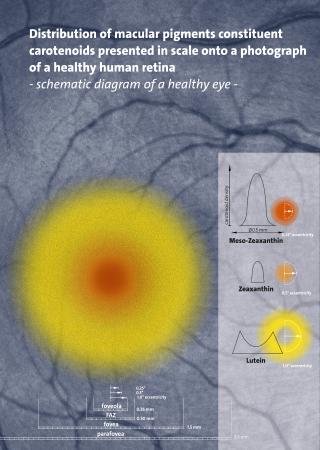
Lutein is a xanthophyll and one of 600 known naturally occurring carotenoids. Lutein is synthesized only by plants, and like other xanthophylls is found in high quantities in green leafy vegetables such as spinach, kale and yellow carrots. In green plants, xanthophylls act to modulate light energy and serve as non-photochemical quenching agents to deal with triplet chlorophyll, an excited form of chlorophyll which is overproduced at very high light levels during photosynthesis. See xanthophyll cycle for this topic.

Zeaxanthin is one of the most common carotenoids in nature, and is used in the xanthophyll cycle. Synthesized in plants and some micro-organisms, it is the pigment that gives paprika, corn, saffron, goji (wolfberries), and many other plants and microbes their characteristic color.

Picrocrocin is a monoterpene glycoside precursor of safranal. It is found in the spice saffron, which comes from the crocus flower. Picrocrocin has a bitter taste, and is the chemical most responsible for the taste of saffron.

Carotenoid oxygenases are a family of enzymes involved in the cleavage of carotenoids to produce, for example, retinol, commonly known as vitamin A. This family includes an enzyme known as RPE65 which is abundantly expressed in the retinal pigment epithelium where it catalyzed the formation of 11-cis-retinol from all-trans-retinyl esters.
CRT is the gene cluster responsible for the biosynthesis of carotenoids. Those genes are found in eubacteria, in algae and are cryptic in Streptomyces griseus.

Damascenones are a series of closely related chemical compounds that are components of a variety of essential oils. The damascenones belong to a family of chemicals known as rose ketones, which also includes damascones and ionones. beta-Damascenone is a major contributor to the aroma of roses, despite its very low concentration, and is an important fragrance chemical used in perfumery.
Non-photochemical quenching (NPQ) is a mechanism employed by plants and algae to protect themselves from the adverse effects of high light intensity. It involves the quenching of singlet excited state chlorophylls (Chl) via enhanced internal conversion to the ground state, thus harmlessly dissipating excess excitation energy as heat through molecular vibrations. NPQ occurs in almost all photosynthetic eukaryotes, and helps to regulate and protect photosynthesis in environments where light energy absorption exceeds the capacity for light utilization in photosynthesis.
Antheraxanthin is a bright yellow accessory pigment found in many organisms that perform photosynthesis. It is a xanthophyll cycle pigment, an oil-soluble alcohol within the xanthophyll subgroup of carotenoids. Antheraxanthin is both a component in and product of the cellular photoprotection mechanisms in photosynthetic green algae, red algae, euglenoids, and plants.

15-cis-phytoene desaturases, are enzymes involved in the carotenoid biosynthesis in plants and cyanobacteria. Phytoene desaturases are membrane-bound enzymes localized in plastids and introduce two double bonds into their colorless substrate phytoene by dehydrogenation and isomerize two additional double bonds. This reaction starts a biochemical pathway involving three further enzymes called the poly-cis pathway and leads to the red colored lycopene. The homologous phytoene desaturase found in bacteria and fungi (CrtI) converts phytoene directly to lycopene by an all-trans pathway.
4,4'-Diapophytoene desaturase is an enzyme with systematic name 15-cis-4,4'-diapophytoene:FAD oxidoreductase. This enzyme catalyses the following chemical reaction
Zeaxanthin epoxidase (EC 1.14.13.90, Zea-epoxidase) is an enzyme with systematic name zeaxanthin,NAD(P)H:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction
Beta-carotene 3-hydroxylase (EC 1.14.13.129, beta-carotene 3,3'-monooxygenase, CrtZ) is an enzyme with systematic name beta-carotene,NADH:oxygen 3-oxidoreductase . This enzyme catalyses the following chemical reaction
Zeaxanthin 7,8-dioxygenase (EC 1.14.99.42, zeaxanthin 7,8(7',8')-cleavage dioxygenase, CsZCD) is an enzyme with systematic name zeaxanthin:oxygen oxidoreductase (7,8-cleaving). This enzyme catalyses the following chemical reaction
Prolycopene isomerase is an enzyme with systematic name 7,9,7',9'-tetracis-lycopene cis-trans-isomerase. This enzyme catalyses the following chemical reaction

meso-Zeaxanthin (3R,3´S-Zeaxanthin) is a xanthophyll carotenoid, as it contains oxygen and hydrocarbons, and is one of the three stereoisomers of zeaxanthin. Of the three stereoisomers, meso-zeaxanthin is the second most abundant in nature after 3R,3´R-zeaxanthin, which is produced by plants and algae. To date, meso-zeaxanthin has been identified in specific tissues of marine organisms and in the macula lutea, also known as the "yellow spot", of the human retina.












