
Chaetomium is a genus of fungi in the Chaetomiaceae family. It is a dematiaceous (dark-walled) mold normally found in soil, air, cellulose and plant debris. According to the Dictionary of the Fungi, there are about 95 species in the widespread genus.

Cochliobolus lunatus is a fungal plant pathogen that can cause disease in humans and other animals. The anamorph of this fungus is known as Curvularia lunata, while C. lunatus denotes the teleomorph or sexual stage. They are, however, the same biological entity. C. lunatus is the most commonly reported species in clinical cases of reported Cochliobolus infection.

Setosphaeria rostrata is a heat tolerant fungus with an asexual reproductive form (anamorph) known as Exserohilum rostratum. This fungus is a common plant pathogen, causing leaf spots as well as crown rot and root rot in grasses. It is also found in soils and on textiles in subtropical and tropical regions. Exserohilum rostratum is one of the 35 Exserohilum species implicated uncommonly as opportunistic pathogens of humans where it is an etiologic agent of sinusitis, keratitis, skin lesions and an often fatal meningoencephalitis. Infections caused by this species are most often seen in regions with hot climates like Israel, India and the southern USA.

Fusarium solani is a species complex of at least 26 closely related filamentous fungi in the division Ascomycota, family Nectriaceae. It is the anamorph of Nectria haematococca. It is a common soil fungus and colonist of plant materials. Fusarium solani is implicated in plant disease as well as human disease notably infection of the cornea of the eye.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.
Chaetomium cupreum is a fungus in the family Chaetomiaceae. It is able to decay in manufactured cellulosic materials, and is known to antagonize a wide range of soil microorganisms. This species is component of the biocontrol agent, Ketomium, a commercial biofungicide. It has also been investigated for use in the production of natural dyes. Chaetomium cupreum is mesophilic and known to occur in harsh environments and can rapidly colonize organic substrates in soil. Laboratory cultures of C. cupreum can be propagated on a range of common growth media including potato dextrose at ambient or higher than ambient temperature producing cottony white colonies with a reddish reverse.

Cladophialophora bantiana is a melanin producing mold known to cause brain abscesses in humans. It is one of the most common causes of systemic phaeohyphomycosis in mammals. Cladophialophora bantiana is a member of the ascomycota and has been isolated from soil samples from around the world.
Coniochaeta hoffmannii, also known as Lecythophora hoffmannii, is an ascomycete fungus that grows commonly in soil. It has also been categorized as a soft-rot fungus capable of bringing the surface layer of timber into a state of decay, even when safeguarded with preservatives. Additionally, it has pathogenic properties, although it causes serious infection only in rare cases. A plant pathogen lacking a known sexual state, C. hoffmannii has been classified as a "dematiaceous fungus" despite its contradictory lack of pigmentation; both in vivo and in vitro, there is no correlation between its appearance and its classification.

Chaetomium globosum is a well-known mesophilic member of the mold family Chaetomiaceae. It is a saprophytic fungus that primarily resides on plants, soil, straw, and dung. Endophytic C. globosum assists in cellulose decomposition of plant cells. They are found in habitats ranging from forest plants to mountain soils across various biomes. C. globosum colonies can also be found indoors and on wooden products.
Thielavia subthermophila is a ubiquitous, filamentous fungus that is a member of the phylum Ascomycota and order Sordariales. Known to be found on plants of arid environments, it is an endophyte with thermophilic properties, and possesses dense, pigmented mycelium. Thielavia subthermophila has rarely been identified as a human pathogen, with a small number of clinical cases including ocular and brain infections. For treatment, antifungal drugs such as amphotericin B have been used topically or intravenously, depending upon the condition.

Cladosporium sphaerospermum is a radiotrophic fungus belonging to the genus Cladosporium and was described in 1886 by Albert Julius Otto Penzig from the decaying leaves and branches of Citrus. It is a dematiaceous (darkly-pigmented) fungus characterized by slow growth and largely asexual reproduction. Cladosporium sphaerospermum consists of a complex of poorly morphologically differentiated, "cryptic" species that share many physiological and ecological attributes. In older literature, all of these sibling species were classified as C. sphaerospermum despite their unique nature. Accordingly, there is confusion in older literature reports on the physiological and habitat regularities of C. sphaerospermum in the strict sense. This fungus is most phylogenetically similar to C. fusiforme. According to modern phylogenetic analyses, the previously synonymized species, Cladosporium langeroni, is a distinct species.
Paecilomyces marquandii is a soil-borne filamentous fungus distributed throughout temperate to tropical latitudes worldwide including forest, grassland, sewage sludge and strongly metal polluted area characterized by high tolerance in heavy metals. Simultaneous toxic action of zinc and alachlor result an increase in uptake of metal in this fungus but disrupts the cell membrane. Paecilomyces marquandii is known to parasitize the mushroom, Cuphophyllus virgineus, in the family, Hygrophoraceae. Paecilomyces marquandii is categorised as a biosafety risk group 1 in Canada and is not thought to be a significant pathogen of humans or animals.

Phialophora verrucosa is a pathogenic, dematiaceous fungus that is a common cause of chromoblastomycosis. It has also been reported to cause subcutaneous phaeohyphomycosis and mycetoma in very rare cases. In the natural environment, it can be found in rotting wood, soil, wasp nests, and plant debris. P. verrucosa is sometimes referred to as Phialophora americana, a closely related environmental species which, along with P. verrucosa, is also categorized in the P. carrionii clade.
Botryotrichum murorum is a common soil and indoor fungus resembling members of the genus Chaetomium. The fungus has no known asexual state, and unlike many related fungi, is intolerant of high heat exhibiting limited growth when incubated at temperatures over 35 °C. In rare cases, the fungus is an opportunistic pathogen of marine animals and humans causing cutaneous and subcutaneous infection.
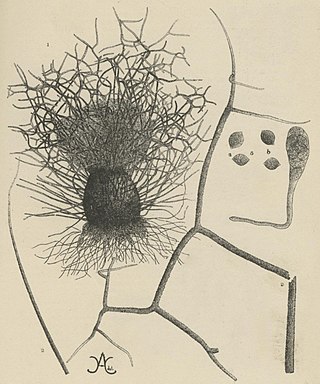
Chaetomium elatum is a very common and widely distributed saprotrophic fungus of the Chaetomiaceae family of molds which has been found to grow on many different substances all over the world. It was first established by Gustav Kunze after he observed it growing on dead leaves. Its defining features that distinguish it from other Chaetomium species are its extremely coarse terminal hairs and the lemon-shaped morphology of its ascospores. It produces many metabolites with potential biotechnology uses including one with promise against the rice blast disease fungus, Magnaporthe grisea. It shows very little pathogenic ability causing confirmed disease in only a few plant species.
Collariella bostrychodes is a fungal decomposer of lignin and carbohydrate in the family Chaetomiaceae commonly found in soil and dung. The fungus is distinguished by a darkened collar-like ostiole around the ostiolar pore, giving the fungus its name. The fungus is highly variable in shape and form, giving raise to the belief that there are two subclades in the species. The ascospores range from lemon-shaped to nearly spherical with slightly pointed ends. It can grow to be pale green and later turn pale bluish grey or olivaceous with age. The fungus produces the toxic secondary metabolite, chaetochromin.
Botryotrichum piluliferum is a fungal species first identified in 1885 by Saccardo and Marchal. It was discovered to be the asexual state of a member of the ascomycete genus, Chaetomium. The name B. piluliferum now applies to the fungus in all its states. B. piluliferum has been found worldwide in a wide range of habitats such as animal dung and vegetation. The colonies of this fungus start off white and grow rapidly to a brown colour. The conidia are smooth and white. B. piluliferum grows optimally at a temperature of 25-30 °C and a pH of 5.5.
Arcopilus aureus is a plant and soil fungus in the genus Arcopilus. It was first identified by A. H. Chivers in 1912, who named it Chaetomium aureum. It was later transferred to the genus Arcopilus by Wang and colleagues. The fungus has recently been recognized to have industrial use for the production of the metabolites resveratrol. and sclerotiorin Additionally, A. aureus has high lead tolerance and clearance, suggesting a potential role in environmental biotechnology.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.
Chaetomium perlucidum is a neurotropic dematiaceous fungus that is naturally found in the soil, including in agricultural soil, and in the stems of dead plants. The fungus can also be found on the feathers of birds, manure, seeds, and even paper. It is able to thrive at temperatures of 35 and 42 °C.








