Adolph Wilhelm Hermann Kolbe was a German chemist and academic, and a major contributor to the birth of modern organic chemistry. He was a professor at Marburg and Leipzig. Kolbe was the first to apply the term synthesis in a chemical context, and contributed to the philosophical demise of vitalism through synthesis of the organic substance acetic acid from carbon disulfide, and also contributed to the development of structural theory. This was done via modifications to the idea of "radicals" and accurate prediction of the existence of secondary and tertiary alcohols, and to the emerging array of organic reactions through his Kolbe electrolysis of carboxylate salts, the Kolbe-Schmitt reaction in the preparation of aspirin and the Kolbe nitrile synthesis. After studies with Wöhler and Bunsen, Kolbe was involved with the early internationalization of chemistry through work in London. He was elected to the Royal Swedish Academy of Sciences, and won the Royal Society of London's Davy Medal in the year of his death. Despite these accomplishments and his training important members of the next generation of chemists, Kolbe is best remembered for editing the Journal für Praktische Chemie for more than a decade, in which his vituperative essays on Kekulé's structure of benzene, van't Hoff's theory on the origin of chirality and Baeyer's reforms of nomenclature were personally critical and linguistically violent. Kolbe died of a heart attack in Leipzig at age 66, six years after the death of his wife, Charlotte.
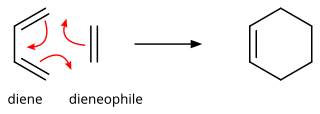
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels–Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.

In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is R−S(=O)2−O−, containing the functional group −S(=O)2−O−, where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
The Kolbe–Schmitt reaction or Kolbe process is a carboxylation chemical reaction that proceeds by treating phenol with sodium hydroxide to form sodium phenoxide, then heating sodium phenoxide with carbon dioxide under pressure, then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid.
The Kolbe electrolysis or Kolbe reaction is an organic reaction named after Hermann Kolbe. The Kolbe reaction is formally a decarboxylative dimerisation of two carboxylic acids. The overall reaction is:
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.

In organic chemistry, the benzoin addition is an addition reaction involving two aldehydes. The reaction generally occurs between aromatic aldehydes or glyoxals, and results in formation of an acyloin. In the classic example, benzaldehyde is converted to benzoin.

Benzoin ( or ) is an organic compound with the formula PhCH(OH)C(O)Ph. It is a hydroxy ketone attached to two phenyl groups. It appears as off-white crystals, with a light camphor-like odor. Benzoin is synthesized from benzaldehyde in the benzoin condensation. It is chiral and it exists as a pair of enantiomers: (R)-benzoin and (S)-benzoin.

In organic chemistry, aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution. Aryl sulfonic acids are used as detergents, dye, and drugs.

Hugo (Ugo) Schiff was an Italian naturalized chemist. The son of a Jewish businessman and brother of the physiologist Moritz Schiff, Hugo Schiff was German by nationality. He discovered Schiff bases and other imines, and was responsible for research into aldehydes; leading to his development of the Schiff test. He also worked in the field of amino acids and the Biuret reagent.

Wilhelm Rudolph Fittig was a German chemist. He discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. Fittig studied the action of sodium on ketones and hydrocarbons. He discovered the Fittig reaction or Wurtz–Fittig reaction for the synthesis of alkylbenzenes, he proposed a diketone structure for benzoquinone and isolated phenanthrene from coal tar. He discovered and synthesized the first lactones and investigated structures of piperine, naphthalene, and fluorene.
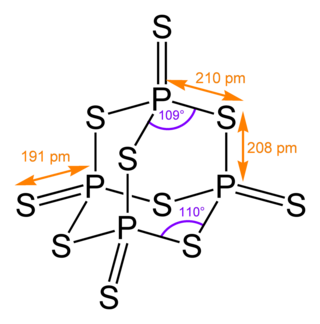
Phosphorus pentasulfide is the inorganic compound with the formula P2S5 (empirical) or P4S10 (molecular). This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF.

The pinacol–pinacolone rearrangement is a method for converting a 1,2-diol to a carbonyl compound in organic chemistry. The 1,2-rearrangement takes place under acidic conditions. The name of the rearrangement reaction comes from the rearrangement of pinacol to pinacolone.

Justus Liebig's Annalen der Chemie was one of the oldest and historically most important journals in the field of organic chemistry worldwide. It was established in 1832 and edited by Justus von Liebig with Friedrich Wöhler and others until Liebig's death in 1873. The journal was originally titled Annalen der Pharmacie; its name was changed to Justus Liebig's Annalen der Chemie in 1874. In its first decades of publishing, the journal was both a periodical containing news of the chemical and pharmaceutical fields and a publisher of primary research. During this time, it was noted to contain rebuttals and criticism of the works it published, inserted by Justus von Liebig during his tenure as an editor. After 1874, changes were made to editorial policies, and the journal published only completed research; later on, in the 20th century, its focus was narrowed to only print articles on organic chemistry, though it had always placed emphasis on the field. The journal was especially influential in the mid-19th century, but by the post-World War II period was considered "no longer as preeminent as it once was".

Nikolay Nikolaevich Zinin was a Russian organic chemist.
Adolph Strecker was a German chemist who is remembered primarily for his work with amino acids.
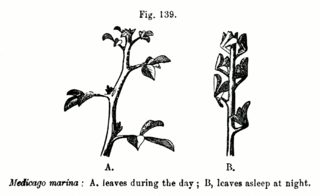
In plant biology, nyctinasty is the circadian rhythm-based nastic movement of higher plants in response to the onset of darkness, or a plant "sleeping". Nyctinastic movements are associated with diurnal light and temperature changes and controlled by the circadian clock. It has been argued that for plants that display foliar nyctinasty, it is a crucial mechanism for survival; however, most plants do not exhibit any nyctinastic movements. Nyctinasty is found in a range of plant species and across xeric, mesic, and aquatic environments, suggesting that this singular behavior may serve a variety of evolutionary benefits. Examples are the closing of the petals of a flower at dusk and the sleep movements of the leaves of many legumes.
Radical theory is an obsolete scientific theory in chemistry describing the structure of organic compounds. The theory was pioneered by Justus von Liebig, Friedrich Wöhler and Auguste Laurent around 1830 and is not related to the modern understanding of free radicals. In this theory, organic compounds were thought to exist as combinations of radicals that could be exchanged in chemical reactions just as chemical elements could be interchanged in inorganic compounds.