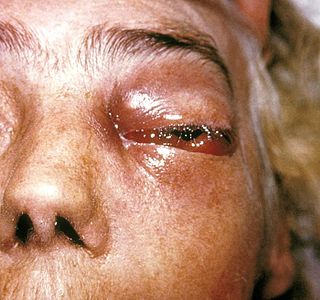
Zygomycosis is the broadest term to refer to infections caused by bread mold fungi of the zygomycota phylum. However, because zygomycota has been identified as polyphyletic, and is not included in modern fungal classification systems, the diseases that zygomycosis can refer to are better called by their specific names: mucormycosis, phycomycosis and basidiobolomycosis. These rare yet serious and potentially life-threatening fungal infections usually affect the face or oropharyngeal cavity. Zygomycosis type infections are most often caused by common fungi found in soil and decaying vegetation. While most individuals are exposed to the fungi on a regular basis, those with immune disorders (immunocompromised) are more prone to fungal infection. These types of infections are also common after natural disasters, such as tornadoes or earthquakes, where people have open wounds that have become filled with soil or vegetative matter.

The Mucorales is the largest and best studied order of zygomycete fungi. Members of this order are sometimes called pin molds. The term mucormycosis is now preferred for infections caused by molds belonging to the order Mucorales.

Mucor is a microbial genus of approximately 40 species of moulds commonly found in soil, digestive systems, plant surfaces, some cheeses like tomme de savoie, rotten vegetable matter and iron oxide residue in the biosorption process.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.

Mucormycosis is any fungal infection caused by fungi in the order Mucorales. Generally, species in the Mucor,Rhizopus,Absidia, and Cunninghamella genera are most often implicated.

Pseudallescheria boydii is a species of fungus classified in the Ascomycota. It is associated with some forms of eumycetoma, maduromycosis and pseudallescheriasis. Typically found in stagnant and polluted water, it has been implicated in the infection of immunocompromised and near-drowned pneumonia patients. Its asexual (anamorphic) form is Scedosporium apiospermum. Treatment of infections with P. boydii is complicated by its resistance to many of the standard antifungal agents normally used to treat infections by filamentous fungi.

The Cunninghamellaceae are a family of fungi in the order Mucorales.
Saksenaea vasiformis is an infectious fungus associated with cutaneous or subcutaneous lesions following trauma. It causes opportunistic infections as the entry of the fungus is through open spaces of cutaneous barrier ranging in severity from mild to severe or fatal. It is found in soils worldwide, but is considered as a rare human pathogen since only 38 cases were reported as of 2012. Saksenaea vasiformis usually fails to sporulate on the routine culture media, creating a challenge for early diagnosis, which is essential for a good prognosis. Infections are usually treated using a combination of amphotericin B and surgery. Saksenaea vasiformis is one of the few fungi known to cause necrotizing fasciitis or "flesh-eating disease".
Rhizomucor pusillus is a species of Rhizomucor. It can cause disease in humans. R. pusillus is a grey mycelium fungi most commonly found in compost piles. Yellow-brown spores grow on a stalk to reproduce more fungal cells.

Majocchi's granuloma is a skin condition characterized by deep, pustular plaques, and is a form of tinea corporis. It is a localized form of fungal folliculitis. Lesions often have a pink and scaly central component with pustules or folliculocentric papules at the periphery. The name comes from Professor Domenico Majocchi, who discovered the disorder in 1883. Majocchi was a professor of dermatology at the University of Parma and later the University of Bologna. The most common dermatophyte is called Trichophyton rubrum.

Apophysomyces variabilis is an emerging fungal pathogen that can cause serious and sometimes fatal infection in humans. This fungus is a soil-dwelling saprobe with tropical to subtropical distribution. It is a zygomycete that causes mucormycosis, an infection in humans brought about by fungi in the order Mucorales. Infectious cases have been reported globally in locations including the Americas, Southeast Asia, India, and Australia. Apophysomyces variabilis infections are not transmissible from person to person.
Coniochaeta hoffmannii, also known as Lecythophora hoffmannii, is an ascomycete fungus that grows commonly in soil. It has also been categorized as a soft-rot fungus capable of bringing the surface layer of timber into a state of decay, even when safeguarded with preservatives. Additionally, it has pathogenic properties, although it causes serious infection only in rare cases. A plant pathogen lacking a known sexual state, C. hoffmannii has been classified as a "dematiaceous fungus" despite its contradictory lack of pigmentation; both in vivo and in vitro, there is no correlation between its appearance and its classification.

Lichtheimia corymbifera is a thermophilic fungus in the phylum Zygomycota. It normally lives as a saprotrophic mold, but can also be an opportunistic pathogen known to cause pulmonary, CNS, rhinocerebral, or cutaneous infections in animals and humans with impaired immunity.

Cunninghamella echinulata is a fungal species in the genus Cunninghamella. It is an asexually reproducing fungus and a mesophile, preferring intermediate temperature ranges. C. echinulata is a common air contaminant, and is currently of interest to the biotechnology industry due to its ability to synthesize γ-linolenic acid as well as its capacity to bioconcentrate metals. This species is a soil saprotroph that forms rhizoids, preferring soils enriched in nitrogen, phosphorus and potassium. It has been reported occasionally an agent of mucormycosis following the inhalation of fungal spores. Czapek's agar is a suitable growth medium for the propagation of C. echinulata.

Rhizopus stolonifer is commonly known as black bread mold. It is a member of Zygomycota and considered the most important species in the genus Rhizopus. It is one of the most common fungi in the world and has a global distribution although it is most commonly found in tropical and subtropical regions. It is a common agent of decomposition of stored foods. Like other members of the genus Rhizopus, R. stolonifer grows rapidly, mostly in indoor environments.
Sarocladium kiliense is a saprobic fungus that is occasionally encountered as a opportunistic pathogen of humans, particularly immunocompromised and individuals. The fungus is frequently found in soil and has been linked with skin and systemic infections. This species is also known to cause disease in the green alga, Cladophora glomerata as well as various fruit and vegetable crops grown in warmer climates.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.
Arthrographis kalrae is an ascomycetous fungus responsible for human nail infections described in 1938 by Cochet as A. langeronii. A. kalrae is considered a weak pathogen of animals including human restricted to the outermost keratinized layers of tissue. Infections caused by this species are normally responsive to commonly used antifungal drugs with only very rare exceptions.
Mycotypha microspora, also known as Microtypha microspora, is a filamentous fungus in the division Zygomycota. It was discovered in a Citrus aurantium peel in 1932 by E. Aline Fenner, who proposed a new genus Mycotypha to accommodate it. Mycotypha africana, which is another species in the genus Mycotypha, is closely related to M. microspora. The fungus has subsequently been isolated from both outdoor and indoor settings around the world, and is typically found in soil and dung. The species rarely causes infections in humans, but has recently been involved in the clinical manifestation of the life-threatening disease mucormycosis.












