Examples of symbioses
Cyanobacteria have been documented to form symbioses with a large range of eukaryotes in both marine and terrestrial environments. Cyanobionts provide benefit through dissolved organic carbon (DOC) production or nitrogen fixation but vary in function depending on their host. [12] Organisms that depend on cyanobacteria often live in nitrogen-limited, oligotrophic environments and can significantly alter marine composition leading to blooms. [12] [13]
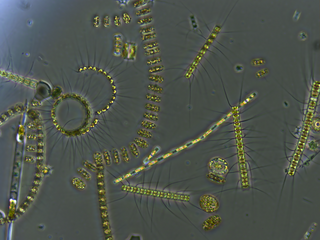
Diatoms
Commonly found in oligotrophic environments, diatoms within the genera Hemiaulus and Rhizosolenia form symbiotic associations with filamentous cyanobacteria in the species Richelia intracellularis. As an endophyte in up to 12 species of Rhizosolenia, R. intracellularis provides fixed nitrogen to its host via the terminally-located heterocyst. [14] Richella-Rhizosolenia symbioses have been found to be abundant within the nitrogen-limited waters of the Central-Pacific Gyre. [15] Several field studies have linked the occurrence of phytoplankton blooms within the gyre to an increase in nitrogen fixation from Richella-Rhizosolenia symbiosis. [14] [15] A dominant organism in warm oligotrophic waters, five species within the genus Hemiaulus receive fixed nitrogen from R. intracellularis. [16] [14] Hemiaulus-Richella symbioses are up to 245 times more abundant than the former, with 80–100% of Hemilalus cells containing the cyanobiont. [17] [18] [19] Nitrogen fixation in the Hemiaulus-Richella symbiosis is 21 to 45 times greater than in the Richella-Rhizosolenia symbiosis within the southwestern Atlantic and Central Pacific Gyre, respectively. [16]
Other genera of diatoms can form symbioses with cyanobacteria; however, their relationships are less known. Nitrogen fixing cyanobacterial symbionts have been found within the diatoms in the genus Epithemia and have been found to possess genes for nitrogen fixation, but have lost genes required for both photosystems and the required pigments to perform photosynthesis. [20] [21] [22]
Dinoflagellates

(a) O. magnificus with numerous cyanobionts present in the upper and lower girdle lists (black arrowheads) of the cingulum termed the symbiotic chamber.
(b) O. steinii with numerous cyanobionts inhabiting the symbiotic chamber.
(c) Enlargement of the area in (b) showing two cyanobionts that are being divided by binary transverse fission (white arrows).
Heterotrophic dinoflagellates can form symbioses with cyanobacteria (phaeosomes), most often in tropical marine environments. [12] The function of the cyanobiont depends on its host species. Abundant marine cyanobacteria in the genus Synechococcus form symbionts with dinoflagellates in the genera Ornithocercus, Histionesis and Citharistes , where it is hypothesized to benefit its host through the provision of fixed nitrogen in oligotrophic, subtropical waters. [24] Increased instances of phaeosome symbiosis have been documented in a stratified, nitrogen-limited environment, and living within a host can provide an anaerobic environment for nitrogen fixation to occur. [25] However, there is conflicting evidence of this. One study on phaeosomes in cells of Ornithocercus spp. has provided evidence that symbionts belonging to the genus Synechococcus, supply organic carbon rather than nitrogen, due to the absence of nitrogenase within these cyanobacteria. [26]
Sponges
One hundred species within the classes Calcarea and Demospongiae form symbioses with cyanobacteria in the genera Aphanocapsa, Synechocystis, Oscillatoria and Phormidium . [12] [27] Cyanobacteria benefit their hosts through providing glycerol and organic phosphates through photosynthesis and supply up to half of their required energy and a majority of their carbon budget. [28] Two groups of sponges with photosynthetic symbionts have been described; these are the "cyanosponges" and "phototrophs". Cyanosponges are mixotrophic and therefore obtain energy through heterotrophic feeding as well as photosynthesis. The latter group receives almost all of their energy requirements through photosynthesis, and therefore have a larger surface area in order increase exposure to sunlight. [29] The most common cyanobionts found in sponges belong to the genus Synechococcus with the species Candidatus Synechococcus spongiarum inhabiting a majority of symbiotic sponges within the Caribbean. [30] Another widely distributed species of cyanobacteria Oscillatoria spongeliae is found within the sponge Lamellodysidea herbacea, alongside ten other species. [27] Oscillatoria spongeliae benefits its host by providing carbon as well as a variety of chlorinated amino derivatives, depending on the host strain. [31]
Lichens
Lichens are the result of a symbiosis between a mycobiont and an autotroph, usually green algae or cyanobacteria. About 8% of lichen species contain a cyanobiont, most commonly members of the genus Nostoc as well as the genera Calothrix, Scytonema and Fischerella. All cyanobionts inhabiting lichens contain heterocysts to fix nitrogen, which can be distributed throughout the host in specific regions (heteromerous) or randomly throughout the thallus (homoiomerous). Additionally, some lichen species are tripartite, containing both a cyanobacterial and green algal symbiont. [32]
Bryophytes
Bryophytes are non-vascular plants encompassing mosses, liverworts, and hornworts, which most often form symbioses with members from the cyanobacterial genus Nostoc . [33] Depending on the host, the cyanobiont can be inside (endophytic) or outside the host (epiphytic). [33] In mosses, cyanobacteria are major nitrogen fixers and grow mostly epiphytically, aside from two species of Sphagnum which protect the cyanobiont from an acidic-bog environment. [34] In terrestrial Arctic environments, cyanobionts are the primary supplier of nitrogen to the ecosystem whether free-living or epiphytic with mosses. [35] Cyanobacterial associations with liverworts are rare, with only four of 340 genera of liverworts harbouring symbionts. [33] Two of the genera, Marchantia and Porella, are epiphytic, while the genera Blasia and Cavicularia are endophytic. [36] In hornworts however, endophytic cyanobionts have been described in more than triple the number of genera relative to liverworts. [37] Bryophytes and their cyanobacterial symbionts possess different structures depending on the nature of the symbiosis. [36] For instance, colonies of cyanobacterial symbionts in the liverwort Blasia spp. are present as auricles (small dots) between the inner and outer papillae near the ventral surface of the liverworts; whereas, cyanobionts in the hornworts Anthoceros and Phaeoceros are present within the thallus', in specialized slime cavities. [33] However, cyanobacteria first must locate and physically interact with their host in order to form a symbiotic relationship. Members of the cyanobacterial genus Nostoc can become motile through the use of hormogonia, while the host plant excretes chemicals to guide the cyanobacteria via chemotaxis. [33] For instance, liverworts in the genus Blasia can secrete HIF, a strong chemo-attractant for nitrogen-starved and symbiotic cyanobacteria. Cells of Nostoc punctiforme , which have been shown to possess genes encoding proteins that complement chemotaxis-related proteins within flowering plants belonging to the genus Gunnera . [38] [39]
Ascidians
Filamentous cyanobacteria within the genera Synechocystis and Prochloron has been found within the tunic cavity of didemnid sea squirts. The symbiosis is proposed to have originated through the intake of a combination of sand and cyanobacteria which eventually proliferated. [40] The hosts benefit from receiving fixed carbon from the cyanobiont while the cyanobiont may benefit by protection from harsh environments. [40] [41]
Echiuroid worms
Little is known about the symbiotic relationship between echiuroid worms and cyanobacteria. Unspecified cyanobacteria have been found within the subepidermal connective tissue of the worms Ikedosoma gogoshimense and Bonellia fuliginosa. [42]
Coral
Unicellular and symbiotic cyanobacteria were discovered in cells of coral belonging to the species Montastraea cavernosa from Caribbean Islands. These cyanobionts coexisted within the symbiotic dinoflagellates zooxanthellae within the corals, and produce the nitrogen-fixing enzyme nitrogenase. [43] Details on the interaction of the symbionts with their hosts remains unknown.