An endosymbiont or endobiont is any organism that lives within the body or cells of another organism most often, though not always, in a mutualistic relationship. This phenomenon is known as endosymbiosis. Examples are nitrogen-fixing bacteria, which live in the root nodules of legumes, single-cell algae inside reef-building corals and bacterial endosymbionts that provide essential nutrients to insects.
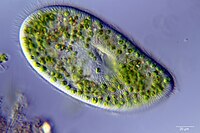
The dinoflagellates are a monophyletic group of single-celled eukaryotes constituting the phylum Dinoflagellata and are usually considered protists. Dinoflagellates are mostly marine plankton, but they also are common in freshwater habitats. Their populations vary with sea surface temperature, salinity, and depth. Many dinoflagellates are photosynthetic, but a large fraction of these are in fact mixotrophic, combining photosynthesis with ingestion of prey.

Kleptoplasty or kleptoplastidy is a process in symbiotic relationships whereby plastids, notably chloroplasts from algae, are sequestered by the host. The word is derived from Kleptes (κλέπτης) which is Greek for thief. The alga is eaten normally and partially digested, leaving the plastid intact. The plastids are maintained within the host, temporarily continuing photosynthesis and benefiting the host.

Cassiopea is a genus of true jellyfish and members of the family Cassiopeidae. They are found in warmer coastal regions around the world, including shallow mangrove swamps, mudflats, canals, and turtle grass flats in Florida, the Caribbean and Micronesia. The medusa usually lives upside-down on the sea floor in shallow areas, which has earned them their common name. These jellyfish partake in a symbiotic relationship with photosynthetic dinoflagellates and therefore, must lie upside-down in areas with sufficient light penetration to fuel their energy source. Where found, there may be numerous individuals with varying shades of white, blue, green and brown.
Symbiotic bacteria are bacteria living in symbiosis with another organism or each other. For example, rhizobia living in root nodules of legumes provide nitrogen fixing activity for these plants.

Symbiodinium is a genus of dinoflagellates that encompasses the largest and most prevalent group of endosymbiotic dinoflagellates known and have photosymbiotic relationships with many species. These unicellular microalgae commonly reside in the endoderm of tropical cnidarians such as corals, sea anemones, and jellyfish, where the products of their photosynthetic processing are exchanged in the host for inorganic molecules. They are also harbored by various species of demosponges, flatworms, mollusks such as the giant clams, foraminifera (soritids), and some ciliates. Generally, these dinoflagellates enter the host cell through phagocytosis, persist as intracellular symbionts, reproduce, and disperse to the environment. The exception is in most mollusks, where these symbionts are intercellular. Cnidarians that are associated with Symbiodinium occur mostly in warm oligotrophic (nutrient-poor), marine environments where they are often the dominant constituents of benthic communities. These dinoflagellates are therefore among the most abundant eukaryotic microbes found in coral reef ecosystems.

The spotted jelly, lagoon jelly, golden medusa, or Papuan jellyfish, is a species of jellyfish from the Indo-Pacific oceans. Like corals, sea anemones, and other sea jellies, it belongs to the phylum Cnidaria. Mastigias papua is one of the numerous marine animals living in symbiosis with zooxanthellae, a photosynthetic alga. Mastigias papua is one of the numerous marine animals living in symbiosis with zooxanthellae, a photosynthetic algae.

Mastigias is a genus of true jellyfish in the family Mastigiidae. It contains seven described species. Members of this genus are found widely in coastal regions of the Indo-Pacific, including saline lakes of Palau, but there are also records from the West Atlantic at Florida and Puerto Rico. The West Atlantic records are most likely the result of accidental introductions by humans.

Hippopus hippopus, also known as the Horse Hoof clam and Strawberry clam, is a species of giant clam in the Subfamily Tridacninae and the genus Hippopus. Hippopus is a delicacy in many Southeast Asian countries due to its high quality meat.

Cassiopea andromeda is one of many cnidarian species called the upside-down jellyfish. It usually lives in intertidal sand or mudflats, shallow lagoons, and around mangroves. This jellyfish, often mistaken for a sea anemone, usually keeps its mouth facing upward. Its yellow-brown bell, which has white or pale streaks and spots, pulsates to run water through its arms for respiration and to gather food.
Polarella is a dinoflagellate, and is the only extant genus of the Suessiaceae family. The genus was described in 1999 by Marina Montresor, Gabriele Procaccini, and Diane K. Stoecker, and contains only one species, Polarella glacialis. Polarella inhabits channels within ice formations in both the Arctic and Antarctic polar regions, where it plays an important role as a primary producer. Polarella is a thecate dinoflagellate, wherein the cell has an outer covering of cellulose plates, which are arranged in nine latitudinal series. The general morphology of Polarella is similar to that of a typical dinoflagellate. and Polarella has a zygotic life history, wherein it alternates between a motile vegetative phase and a non-motile spiny cyst. While it is thought that the cysts of Polarella have lost their ability to form fossils, the cyst life cycle stage has acted as link to extinct members of the Suessiaceae family.
A mixotroph is an organism that can use a mix of different sources of energy and carbon, instead of having a single trophic mode on the continuum from complete autotrophy at one end to heterotrophy at the other. It is estimated that mixotrophs comprise more than half of all microscopic plankton. There are two types of eukaryotic mixotrophs: those with their own chloroplasts, and those with endosymbionts—and those that acquire them through kleptoplasty or through symbiotic associations with prey or enslavement of their organelles.

Corculum cardissa, the heart cockle, is a species of marine bivalve mollusc in the family Cardiidae. It is found in the Indo-Pacific region. It has a symbiotic relationship with dinoflagellates (zooxanthellae), which live within its tissues.
Dinoxanthin is a type of xanthophyll found in dinoflagellates. This compound is a potential antioxidant and may help to protect dinoflagellates against reactive oxygen species.

Orbicella faveolata, commonly known as mountainous star coral, is a colonial stony coral in the family Merulinidae. Orbicella faveolata is native to the coral coast of the Caribbean Sea and the Gulf of Mexico and is listed as "endangered" by the International Union for Conservation of Nature. O. faveolata was formerly known as Montastraea faveolata.
Durusdinium trenchii is an endosymbiotic dinoflagellate, a unicellular alga which commonly resides in the tissues of tropical corals. It has a greater tolerance to fluctuations in water temperatures than do other species in the genus. It was named for the marine biologist R. K. Trench.

Anthopleura ballii, commonly known as the red speckled anemone, is a species of sea anemone in the family Actiniidae. It is found in shallow water in the northeastern Atlantic Ocean.
Robert Kent Trench was an American Biologist who was a professor at the University of California, Santa Barbara. His research considered corals and symbiotic algae, with a focus on the adaption of zooxanthellae. He was awarded the 1994 International Society of Endocytobiology Miescher-Ishida Prize.

Symbiodiniaceae is a family of marine dinoflagellates notable for their symbiotic associations with reef-building corals, sea anemones, jellyfish, marine sponges, giant clams, acoel flatworms, and other marine invertebrates. Symbiotic Symbiodiniaceae are sometimes colloquially referred to as Zooxanthellae, though the latter term can be interpreted to include other families of symbiotic algae as well. While many Symbiodiniaceae species are endosymbionts, others are free living in the water column or sediment.
Miguel Mies is a Brazilian academic, oceanographer, and researcher. He is currently a professor at the Oceanographic Institute of the University of São Paulo (IO-USP) and leads the Coral Reefs and Climate Change Laboratory (LARC). He also serves as the research coordinator for the Coral Vivo Project and is the vice president of the Coral Vivo Institute.