History
The first recorded observation of a cytolethal-distending toxin was in 1987 in a pathogenic strain in E. coli isolated from a young patient. [3] Later that year, scientists W.M. Johnson and H. Lior published the journal article "Production of Shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella spp." in Microbiology Letters. [1] The discovery of other bacteria producing CDT toxins continues to this day.
In 1994 Scott and Kaper cloned and sequenced a cdt operon from another E. coli strain, publishing in Infection and Immunity. [1] [5] The three genes discovered were denoted cdtA, cdtB, and cdtC. [5]
In 1997, the first paper of many to show G2/M cell cycle arrest caused by a cytolethal distending toxin was published in Molecular Microbiology . [1] The study focused on another E. coli strain. This paper was followed by a 1999 publication in Infectious Immunity, which demonstrated that H. ducreyi CDT causes cell death via apoptosis. This finding was also confirmed for other cytolethal distending toxins in subsequent studies.
The discovery of the homology of cdtB to mammalian DNase I and the current AB model for the toxin were published in early 2000. [2] [6] Further research and the publication of crystal structures for the CDT toxins from two different species continues to support this model. [1]
Cellular effects
CDT toxins are genotoxins capable of directly damaging DNA in target cells. They are the only AB-type toxins discovered that display DNase activity, allowing them to introduce breaks into the target cell's DNA. [1] [4]
In many cell lines including human fibroblasts, epithelial cells, endothelial cells, and keratinocytes, CDTs cause G2/M cell cycle arrest, cytoplasmic distension, and eventual cell death via apoptosis. [1] [3] [8] Most publications attribute the G2/M cycle arrest to the buildup of irreversible DNA damage from the toxin's DNase activity as the trigger for the G2/M cell cycle arrest, but other research suggests that this model is incomplete. [8] The cytoplasmic distension is a direct result of the G2/M cell cycle arrest. The cell enlarges in preparation for mitosis, but cannot divide to restore its normal size. Aside from classical apoptosis, signs of cellular senescence has also been observed in normal and cancer cell lines (fibroblasts, HeLa and U2-OS) after CDT intoxication [9]
In lymphocytes, cell death occurs quickly and is not preceded by significant cytoplasmic distension. [8] The ability of these toxins to effect lymphocytes differently may be advantageous to the bacteria that utilize these toxins, but the mechanism behind this phenomenon is not yet well understood.
Toxin structure
The active, assembled toxin is a tripartite structure with three distinct subunits- CdtA, CdtB, and CdtC. In terms of function, it is an AB toxin. In this context, the CdtB subunit is actually the catalytically active "A" subunit, and the CdtA and CdtC together form the binding "B" subunit, which helps the toxin bind and enter target cells. [6] Some literature refers to the toxin structure as AB2 to reflect the presence of both CdtA and CdtC.
Different from all other CDTs, Salmonella enterica serovar Typhi CDT (SeCDT) has no CdtA and CdtC homologues. However, encoded closely to the active subunit cdtb, the Pertussis-like toxin A and B (pltA/pltB) have been shown to be essential for cellular intoxication. [10] PltA and PltB have a different structure from CdtA and CdtC, thus promoting CdtB activity in a different way. Both PltA and PltB have been found to bind directly to CdtB in vitro. [10] In addition, different from all other CDTs, Salmonella genotoxin is produced only upon bacterial internalization in infected cells, thus the SeCDT traffic may differ remarkably from the canonical ones.

Shiga toxins are a family of related toxins with two major groups, Stx1 and Stx2, expressed by genes considered to be part of the genome of lambdoid prophages. The toxins are named after Kiyoshi Shiga, who first described the bacterial origin of dysentery caused by Shigella dysenteriae. Shiga-like toxin (SLT) is a historical term for similar or identical toxins produced by Escherichia coli. The most common sources for Shiga toxin are the bacteria S. dysenteriae and some serotypes of Escherichia coli (STEC), which includes serotypes O157:H7, and O104:H4.

FtsZ is a protein encoded by the ftsZ gene that assembles into a ring at the future site of bacterial cell division. FtsZ is a prokaryotic homologue of the eukaryotic protein tubulin. The initials FtsZ mean "Filamenting temperature-sensitive mutant Z." The hypothesis was that cell division mutants of E. coli would grow as filaments due to the inability of the daughter cells to separate from one another. FtsZ is found in almost all bacteria, many archaea, all chloroplasts and some mitochondria, where it is essential for cell division. FtsZ assembles the cytoskeletal scaffold of the Z ring that, along with additional proteins, constricts to divide the cell in two.
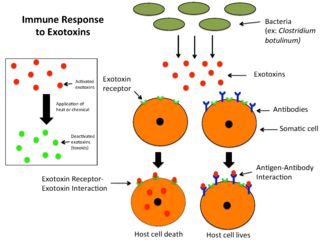
An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.

The SOS response is a global response to DNA damage in which the cell cycle is arrested and DNA repair and mutagenesis is induced. The system involves the RecA protein. The RecA protein, stimulated by single-stranded DNA, is involved in the inactivation of the repressor (LexA) of SOS response genes thereby inducing the response. It is an error-prone repair system that contributes significantly to DNA changes observed in a wide range of species.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.
DNA gyrase, or simply gyrase, is an enzyme within the class of topoisomerase and is a subclass of Type II topoisomerases that reduces topological strain in an ATP dependent manner while double-stranded DNA is being unwound by elongating RNA-polymerase or by helicase in front of the progressing replication fork. It is the only known enzyme to actively contribute negative supercoiling to DNA, while it also is capable of relaxing positive supercoils. It does so by looping the template to form a crossing, then cutting one of the double helices and passing the other through it before releasing the break, changing the linking number by two in each enzymatic step. This process occurs in bacteria, whose single circular DNA is cut by DNA gyrase and the two ends are then twisted around each other to form supercoils. Gyrase is also found in eukaryotic plastids: it has been found in the apicoplast of the malarial parasite Plasmodium falciparum and in chloroplasts of several plants. Bacterial DNA gyrase is the target of many antibiotics, including nalidixic acid, novobiocin, albicidin, and ciprofloxacin.

Lysogeny, or the lysogenic cycle, is one of two cycles of viral reproduction. Lysogeny is characterized by integration of the bacteriophage nucleic acid into the host bacterium's genome or formation of a circular replicon in the bacterial cytoplasm. In this condition the bacterium continues to live and reproduce normally, while the bacteriophage lies in a dormant state in the host cell. The genetic material of the bacteriophage, called a prophage, can be transmitted to daughter cells at each subsequent cell division, and later events can release it, causing proliferation of new phages via the lytic cycle. Lysogenic cycles can also occur in eukaryotes, although the method of DNA incorporation is not fully understood. For instance the AIDS viruses can either infect humans lytically, or lay dormant (lysogenic) as part of the infected cells' genome, keeping the ability to return to lysis at a later time. The rest of this article is about lysogeny in bacterial hosts.
Casein kinase 2 (CK2/CSNK2) is a serine/threonine-selective protein kinase that has been implicated in cell cycle control, DNA repair, regulation of the circadian rhythm, and other cellular processes. De-regulation of CK2 has been linked to tumorigenesis as a potential protection mechanism for mutated cells. Proper CK2 function is necessary for survival of cells as no knockout models have been successfully generated.
The AB5 toxins are six-component protein complexes secreted by certain pathogenic bacteria known to cause human diseases such as cholera, dysentery, and hemolytic–uremic syndrome. One component is known as the A subunit, and the remaining five components are B subunits. All of these toxins share a similar structure and mechanism for entering targeted host cells. The B subunit is responsible for binding to receptors to open up a pathway for the A subunit to enter the cell. The A subunit is then able to use its catalytic machinery to take over the host cell's regular functions.

ADP-ribosylation is the addition of one or more ADP-ribose moieties to a protein. It is a reversible post-translational modification that is involved in many cellular processes, including cell signaling, DNA repair, gene regulation and apoptosis. Improper ADP-ribosylation has been implicated in some forms of cancer. It is also the basis for the toxicity of bacterial compounds such as cholera toxin, diphtheria toxin, and others.

The AB toxins are two-component protein complexes secreted by a number of pathogenic bacteria, though there is a pore-forming AB toxin found the eggs of a snail. They can be classified as Type III toxins because they interfere with internal cell function. They are named AB toxins due to their components: the "A" component is usually the "active" portion, and the "B" component is usually the "binding" portion. The "A" subunit possesses enzyme activity, and is transferred to the host cell following a conformational change in the membrane-bound transport "B" subunit. These proteins consist of two independent polypeptides, which correspond to the A/B subunit moieties. The enzyme component (A) enters the cell through endosomes produced by the oligomeric binding/translocation protein (B), and prevents actin polymerisation through ADP-ribosylation of monomeric G-actin.

A circular chromosome is a chromosome in bacteria, archaea, mitochondria, and chloroplasts, in the form of a molecule of circular DNA, unlike the linear chromosome of most eukaryotes.

Caspase-activated DNase (CAD) or DNA fragmentation factor subunit beta is a protein that in humans is encoded by the DFFB gene. It breaks up the DNA during apoptosis and promotes cell differentiation. It is usually an inactive monomer inhibited by ICAD. This is cleaved before dimerization.

A toxin-antitoxin system consists of a "toxin" and a corresponding "antitoxin", usually encoded by closely linked genes. The toxin is usually a protein while the antitoxin can be a protein or an RNA. Toxin-antitoxin systems are widely distributed in prokaryotes, and organisms often have them in multiple copies. When these systems are contained on plasmids – transferable genetic elements – they ensure that only the daughter cells that inherit the plasmid survive after cell division. If the plasmid is absent in a daughter cell, the unstable antitoxin is degraded and the stable toxic protein kills the new cell; this is known as 'post-segregational killing' (PSK).

Escherichia coli is a gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms (endotherms). Most E. coli strains are harmless, but pathogenic varieties cause serious food poisoning, septic shock, meningitis, or urinary tract infections in humans. Unlike normal flora E. coli, the pathogenic varieties produce toxins and other virulence factors that enable them to reside in parts of the body normally not inhabited by E. coli, and to damage host cells. These pathogenic traits are encoded by virulence genes carried only by the pathogens.
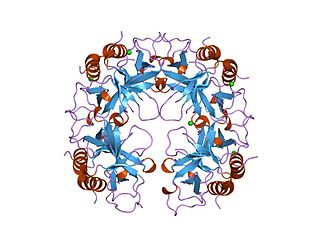
The CcdA/CcdB Type II Toxin-antitoxin system is one example of the bacterial toxin-antitoxin (TA) systems that encode two proteins, one a potent inhibitor of cell proliferation (toxin) and the other its specific antidote (antitoxin). These systems preferentially guarantee growth of plasmid-carrying daughter cells in a bacterial population by killing newborn bacteria that have not inherited a plasmid copy at cell division.
Campylobacter coli is a Gram-negative, microaerophilic, non-endospore-forming, S-shaped bacterial species within genus Campylobacter. In humans, it C. coli can cause campylobacteriosis, a diarrhoeal disease which is the most frequently reported foodborne illness in the European Union. C. coli grows slowly with an optimum temperature of 42 °C. When exposed to air for long periods, they become spherical or coccoid shaped.
Ribonuclease E is a bacterial ribonuclease that participates in the processing of ribosomal RNA and the chemical degradation of bulk cellular RNA.
Contact-dependent growth inhibition (CDI) is a phenomenon where a bacterial cell may deliver a polymorphic toxin molecule into neighbouring bacterial cells upon direct cell-cell contact, causing growth arrest or cell death.
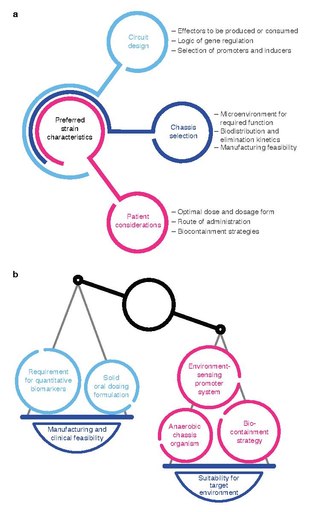
Bacterial therapy is the therapeutic use of bacteria to treat diseases. Bacterial therapeutics are living medicines, and may be wild type bacteria or bacteria that have been genetically engineered to possess therapeutic properties that is injected into a patient. Other examples of living medicines include cellular therapeutics, activators of anti-tumor immunity, or synergizing with existing tools and approaches. and phage therapeutics, or as delivery vehicles for treatment, diagnosis, or imaging, complementing or synergizing with existing tools and approaches.














