
Ketamine is a dissociative anesthetic used medically for induction and maintenance of anesthesia. It is also used as a treatment for depression and in pain management. Ketamine is an NMDA receptor antagonist which accounts for most of its psychoactive effects.
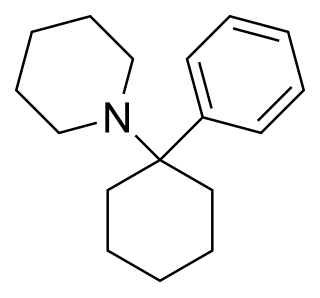
Phencyclidine or phenylcyclohexyl piperidine (PCP), also known in its use as a street drug as angel dust among other names, is a dissociative anesthetic mainly used recreationally for its significant mind-altering effects. PCP may cause hallucinations, distorted perceptions of sounds, and violent behavior. As a recreational drug, it is typically smoked, but may be taken by mouth, snorted, or injected. It may also be mixed with cannabis or tobacco.

N-methyl-D-aspartic acid or N-methyl-D-aspartate (NMDA) is an amino acid derivative that acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor. Unlike glutamate, NMDA only binds to and regulates the NMDA receptor and has no effect on other glutamate receptors. NMDA receptors are particularly important when they become overactive during, for example, withdrawal from alcohol as this causes symptoms such as agitation and, sometimes, epileptiform seizures.

The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a "coincidence detector" and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

Dizocilpine (INN), also known as MK-801, is a pore blocker of the NMDA receptor, a glutamate receptor, discovered by a team at Merck in 1982. Glutamate is the brain's primary excitatory neurotransmitter. The channel is normally blocked with a magnesium ion and requires depolarization of the neuron to remove the magnesium and allow the glutamate to open the channel, causing an influx of calcium, which then leads to subsequent depolarization. Dizocilpine binds inside the ion channel of the receptor at several of PCP's binding sites thus preventing the flow of ions, including calcium (Ca2+), through the channel. Dizocilpine blocks NMDA receptors in a use- and voltage-dependent manner, since the channel must open for the drug to bind inside it. The drug acts as a potent anti-convulsant and probably has dissociative anesthetic properties, but it is not used clinically for this purpose because of the discovery of brain lesions, called Olney's lesions (see below), in laboratory rats. Dizocilpine is also associated with a number of negative side effects, including cognitive disruption and psychotic-spectrum reactions. It inhibits the induction of long term potentiation and has been found to impair the acquisition of difficult, but not easy, learning tasks in rats and primates. Because of these effects of dizocilpine, the NMDA receptor pore blocker ketamine is used instead as a dissociative anesthetic in human medical procedures. While ketamine may also trigger temporary psychosis in certain individuals, its short half-life and lower potency make it a much safer clinical option. However, dizocilpine is the most frequently used uncompetitive NMDA receptor antagonist in animal models to mimic psychosis for experimental purposes.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.

The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.

Glutamate receptors are synaptic and non synaptic receptors located primarily on the membranes of neuronal and glial cells. Glutamate is abundant in the human body, but particularly in the nervous system and especially prominent in the human brain where it is the body's most prominent neurotransmitter, the brain's main excitatory neurotransmitter, and also the precursor for GABA, the brain's main inhibitory neurotransmitter. Glutamate receptors are responsible for the glutamate-mediated postsynaptic excitation of neural cells, and are important for neural communication, memory formation, learning, and regulation.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for humans and animals; the state of anesthesia they induce is referred to as dissociative anesthesia.

N-Acetylaspartylglutamic acid is a peptide neurotransmitter and the third-most-prevalent neurotransmitter in the mammalian nervous system. NAAG consists of N-acetylaspartic acid (NAA) and glutamic acid coupled via a peptide bond.
The glutamate hypothesis of schizophrenia models the subset of pathologic mechanisms of schizophrenia linked to glutamatergic signaling. The hypothesis was initially based on a set of clinical, neuropathological, and, later, genetic findings pointing at a hypofunction of glutamatergic signaling via NMDA receptors. While thought to be more proximal to the root causes of schizophrenia, it does not negate the dopamine hypothesis, and the two may be ultimately brought together by circuit-based models. The development of the hypothesis allowed for the integration of the GABAergic and oscillatory abnormalities into the converging disease model and made it possible to discover the causes of some disruptions.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.
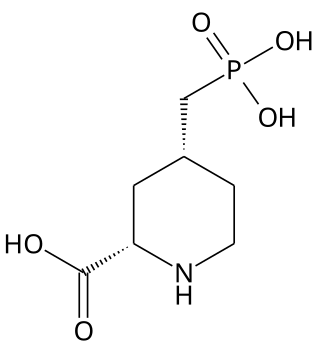
Selfotel (CGS-19755) is a drug which acts as a competitive NMDA antagonist, directly competing with glutamate for binding to the receptor. Initial studies showed it to have anticonvulsant, anxiolytic, analgesic and neuroprotective effects, and it was originally researched for the treatment of stroke, but subsequent animal and human studies showed phencyclidine-like effects, as well as limited efficacy and evidence for possible neurotoxicity under some conditions, and so clinical development was ultimately discontinued.

2-Methyl-6-(phenylethynyl)pyridine (MPEP) is a research drug which was one of the first compounds found to act as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. After being originally patented as a liquid crystal for LCDs, it was developed by the pharmaceutical company Novartis in the late 1990s. It was found to produce neuroprotective effects following acute brain injury in animal studies, although it was unclear whether these results were purely from mGluR5 blockade as it also acts as a weak NMDA antagonist, and as a positive allosteric modulator of another subtype mGlu4, and there is also evidence for a functional interaction between mGluR5 and NMDA receptors in the same populations of neurons. It was also shown to produce antidepressant and anxiolytic effects in animals, and to reduce the effects of morphine withdrawal, most likely due to direct interaction between mGluR5 and the μ-opioid receptor.

LY-379,268 is a drug that is used in neuroscience research, which acts as a potent and selective agonist for the group II metabotropic glutamate receptors (mGluR2/3).

Licostinel (INN) is a competitive, silent antagonist of the glycine site of the NMDA receptor. It was under investigation by Acea Pharmaceuticals as a neuroprotective agent for the treatment of cerebral ischemia associated with stroke and head injuries but was ultimately never marketed. In clinical trials, licostinel did not produce phencyclidine-like psychotomimetic effects at the doses tested, though transient sedation, dizziness, and nausea were observed. In addition to its actions at the NMDA receptor, licostinel also acts as an antagonist of the AMPA and kainate receptors at high concentrations.

PD-137889 (N-methylhexahydrofluorenamine) is a chemical compound that is active as an NMDA receptor antagonist in the central nervous system at roughly 30 times the potency of the "flagship" of its class, ketamine, and substitutes for phencyclidine in animal studies. Ki [3H]TCP binding = 27 nM versus ketamine's Ki = 860 nM.

Mark Lee Mayer is scientist emeritus at the National Institutes of Health (NIH). His research investigates glutamate receptor ion channels, the major mediators of excitatory synapses in the brain. He has made numerous observations that have changed our view of receptor function and neurotransmission in the brain. Major findings include discovery of the block of NMDA receptors by extracellular Mg and their high Ca permeability; analysis of the permeation and block of Ca permeable AMPA and kainate receptors by cytoplasmic polyamines; and structural studies on ligand binding, allosteric modulation, and gating using X-ray diffraction and cryoelectron microscopy.

Bita Moghaddam is an Iranian-American neuroscientist and author. She is currently the Ruth Matarazzo Professor of Behavioral Neuroscience at Oregon Health & Science University. Moghaddam investigates the neuronal processes underlying emotion and cognition as a first step to designing strategies to treat and prevent brain illnesses.

Willardiine (correctly spelled with two successive i's) or (S)-1-(2-amino-2-carboxyethyl)pyrimidine-2,4-dione is a chemical compound that occurs naturally in the seeds of Mariosousa willardiana and Acacia sensu lato. The seedlings of these plants contain enzymes capable of complex chemical substitutions that result in the formation of free amino acids (See:#Synthesis). Willardiine is frequently studied for its function in higher level plants. Additionally, many derivates of willardiine are researched for their potential in pharmaceutical development. Willardiine was first discovered in 1959 by R. Gmelin, when he isolated several free, non-protein amino acids from Acacia willardiana (another name for Mariosousa willardiana) when he was studying how these families of plants synthesize uracilyalanines. A related compound, Isowillardiine, was concurrently isolated by a different group, and it was discovered that the two compounds had different structural and functional properties. Subsequent research on willardiine has focused on the functional significance of different substitutions at the nitrogen group and the development of analogs of willardiine with different pharmacokinetic properties. In general, Willardiine is the one of the first compounds studied in which slight changes to molecular structure result in compounds with significantly different pharmacokinetic properties.



















