
In organic chemistry, an allyl group is a substituent with the structural formula −CH2−HC=CH2. It consists of a methylene bridge attached to a vinyl group. The name is derived from the scientific name for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.
Amination is the process by which an amine group is introduced into an organic molecule. This type of reaction is important because organonitrogen compounds are pervasive.

Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known.
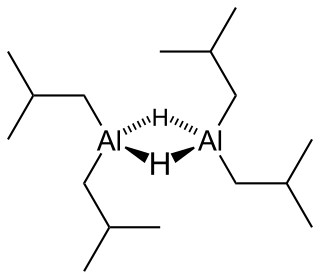
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis.
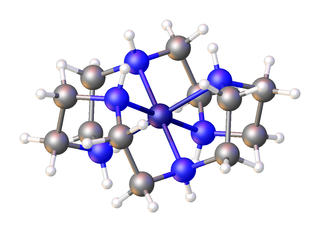
In organic chemistry, an aza-crown ether is an aza analogue of a crown ether. That is, it has a nitrogen atom in place of each oxygen atom around the ring. While the parent crown ethers have the formulae (CH2CH2O)n, the parent aza-crown ethers have the formulae (CH2CH2NH)n, where n = 3, 4, 5, 6. Well-studied aza crowns include triazacyclononane, cyclen, and hexaaza-18-crown-6.
![<span class="mw-page-title-main">Eschenmoser's salt</span> Ionic compound with the formula [(H3C–)2N–CH2]I](https://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Eschenmosersalz.png/320px-Eschenmosersalz.png)
In organic chemistry, Eschenmoser's salt is the ionic, organic compound [(CH3)2NCH2]I. It is the iodide salt of the dimethylaminomethylene cation [(CH3)2NCH2]+.

Allylamine is an organic compound with the formula C3H5NH2. This colorless liquid is the simplest stable unsaturated amine.

n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Its vapours are heavier than air and it produces toxic oxides of nitrogen during combustion.

tert-Butylamine (also erbumine and other names) is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. tert-Butylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and isobutylamine.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.

Triisobutylaluminium (TiBA) is an organoaluminium compound with the formula Al(CH2CH(CH3)2)3. This colorless pyrophoric liquid is mainly used to make linear primary alcohols and α-olefins.
In organic chemistry, ethenolysis is a chemical process in which internal olefins are degraded using ethylene as the reagent. The reaction is an example of cross metathesis. The utility of the reaction is driven by the low cost of ethylene as a reagent and its selectivity. It produces compounds with terminal alkene functional groups (α-olefins), which are more amenable to other reactions such as polymerization and hydroformylation.

Methional is an organic compound with the formula CH3SCH2CH2CHO. It is a colorless liquid that is a degradation product of methionine. It is a notable flavor in potato-based snacks, namely potato chips, one of the most popular foods containing methional. Traces of the compound can also be found in black tea and green tea based products. Methional contains both aldehyde and thioether functional groups. It is readily soluble in alcohol solvents, including propylene glycol and dipropylene glycol.
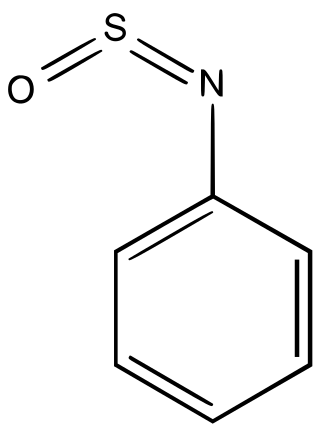
Sulfinylamines are organosulfur compounds with the formula RNSO where R = an organic substituent. These compounds are, formally speaking, derivatives of HN=S=O, i.e. analogues of sulfur dioxide and of sulfur diimide. A common example is N-sulfinylaniline. Sulfinyl amines are dienophile. They undergo [2+2] cycloaddition to ketenes.
1,5-Hexadiene is the organic compound with the formula (CH2)2(CH=CH2)2. It is a colorless, volatile liquid. It is used as a crosslinking agent and precursor to a variety of other compounds.
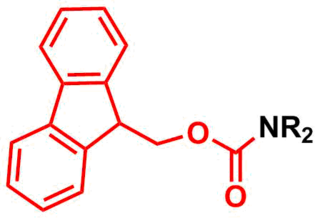
The fluorenylmethoxycarbonyl protecting group (Fmoc) is a base-labile protecting group used in organic synthesis.
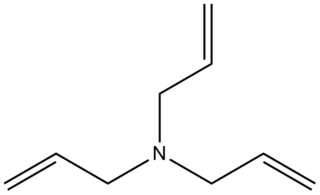
Triallylamine is the organic compound with the formula N(CH2CH=CH2)3. It is a colorless liquid with an ammonia-like odor. It is multifunctional, featuring a tertiary amine and three alkene groups. Triallylamine (and mono- and diallyl amines) is produced by the treating allyl chloride with ammonia.










![<span class="mw-page-title-main">Eschenmoser's salt</span> Ionic compound with the formula [(H3C–)2N–CH2]I](https://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Eschenmosersalz.png/320px-Eschenmosersalz.png)







