In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.
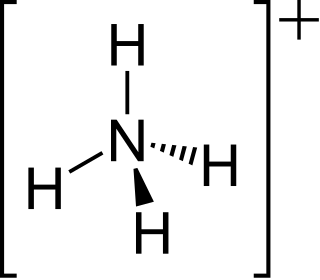
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one less carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.
Morpholine is an organic chemical compound having the chemical formula O(CH2CH2)2NH. This heterocycle features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium. For example, treating morpholine with hydrochloric acid makes the salt morpholinium chloride. It is a colorless liquid with a weak, ammonia- or fish-like odor. The naming of morpholine is attributed to Ludwig Knorr, who incorrectly believed it to be part of the structure of morphine.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered the most important way to make amines, and a majority of amines made in the pharmaceutical industry are made this way.
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with ammonia in the presence of potassium cyanide. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used commercially for the production of racemic methionine from methional.
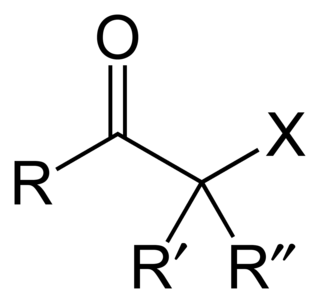
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.

Allylamine is an organic compound with the formula C3H5NH2. This colorless liquid is the simplest stable unsaturated amine.
The Leuckart reaction is the chemical reaction that converts aldehydes or ketones to amines by reductive amination in the presence of heat. The reaction, named after Rudolf Leuckart, uses either ammonium formate or formamide as the nitrogen donor and reducing agent. It requires high temperatures, usually between 120 and 130 °C; for the formamide variant, the temperature can be greater than 165 °C.
The Kornblum–DeLaMare rearrangement is a rearrangement reaction in organic chemistry in which a primary or secondary organic peroxide is converted to the corresponding ketone and alcohol under acid or base catalysis. The reaction is relevant as a tool in organic synthesis and is a key step in the biosynthesis of prostaglandins.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).
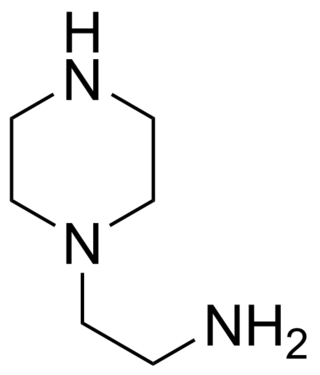
Aminoethylpiperazine (AEP) is a derivative of piperazine. This ethyleneamine contains three nitrogen atoms; one primary, one secondary and one tertiary. It is a corrosive organic liquid and can cause second or third degree burns. Aminoethylpiperazine can also cause pulmonary edema as a result of inhalation. It is REACH and TSCA registered.
Amine alkylation (amino-dehalogenation) is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution, and the reaction product is a higher substituted amine. The method is widely used in the laboratory, but less so industrially, where alcohols are often preferred alkylating agents.

An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application, oxaziridines are intermediates in the industrial production of hydrazine. Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions. Oxaziridines also serve as precursors to amides and participate in [3+2] cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Chiral oxaziridine derivatives effect asymmetric oxygen transfer to prochiral enolates as well as other substrates. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center.
In chemistry, ammonolysis (/am·mo·nol·y·sis/) is the process of splitting ammonia into . Ammonolysis reactions can be conducted with organic compounds to produce amines (molecules containing a nitrogen atom with a lone pair, :N), or with inorganic compounds to produce nitrides. This reaction is analogous to hydrolysis in which water molecules are split. Similar to water, liquid ammonia also undergoes auto-ionization, , where the rate constant is k = 1.9 × 10-38.
Diallylamine is the organic compound with the formula HN(CH2CH=CH2)2. It is a colorless liquid with an ammonia-like odor. It is multifunctional, featuring a secondary amine and two alkene groups. Diallylamine is used in the production of N,N-diallyldichloroacetamide and N,N-diallyldimethylammonium chloride.












