 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Dimethyl dicarbonate | |
| Other names DMDC; Dicarbonic acid dimethyl ester; Dimethyl pyrocarbonate; Velcorin | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.022.601 |
| EC Number |
|
| E number | E242 (preservatives) |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
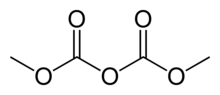
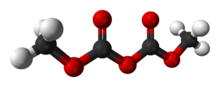
| C4H6O5 | |
| Molar mass | 134.087 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.25 g/mL |
| Melting point | 16 to 18 °C (61 to 64 °F; 289 to 291 K) |
| Boiling point | 172 °C (342 °F; 445 K) |
| Viscosity | 2.1 Pa·s (20 °C) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Toxic |
| GHS labelling: | |
    | |
| Danger | |
| H226, H302, H312, H314, H330 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P270, P271, P280, P284, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P320, P321, P322, P330, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Related compounds | |
Related compounds | Di-tert-butyl dicarbonate diethylpyrocarbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dimethyl dicarbonate (DMDC) is a colorless liquid with a pungent odor at high concentration at room temperature. It is primarily used as a beverage preservative, processing aid, or sterilant (INS No. 242) being highly active against typical beverage spoiling microorganisms like yeast, bacteria, or mould. [1]