
A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.

Cathepsins are proteases found in all animals as well as other organisms. There are approximately a dozen members of this family, which are distinguished by their structure, catalytic mechanism, and which proteins they cleave. Most of the members become activated at the low pH found in lysosomes. Thus, the activity of this family lies almost entirely within those organelles. There are, however, exceptions such as cathepsin K, which works extracellularly after secretion by osteoclasts in bone resorption. Cathepsins have a vital role in mammalian cellular turnover.
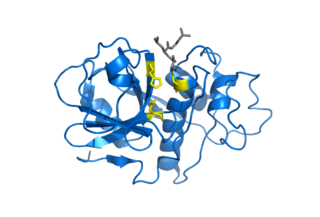
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.

Cathepsin S is a protein that in humans is encoded by the CTSS gene. Transcript variants utilizing alternative polyadenylation signals exist for this gene.

Cathepsin C (CTSC) also known as dipeptidyl peptidase I (DPP-I) is a lysosomal exo-cysteine protease belonging to the peptidase C1 protein family, a subgroup of the cysteine cathepsins. In humans, it is encoded by the CTSC gene.
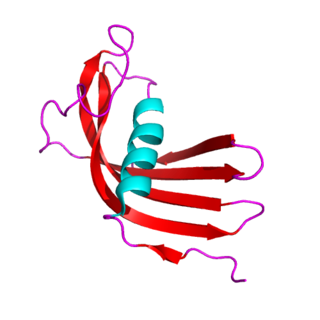
The cystatins are a family of cysteine protease inhibitors which share a sequence homology and a common tertiary structure of an alpha helix lying on top of an anti-parallel beta sheet. The family is subdivided as described below.

Cathepsin B belongs to a family of lysosomal cysteine proteases known as the cysteine cathepsins and plays an important role in intracellular proteolysis. In humans, cathepsin B is encoded by the CTSB gene. Cathepsin B is upregulated in certain cancers, in pre-malignant lesions, and in various other pathological conditions.

Actinidain is a type of cysteine protease enzyme found in fruits including kiwifruit, pineapple, mango, banana, figs, and papaya. This enzyme is part of the peptidase C1 family of papain-like proteases.

Cathepsin L1 is a protein that in humans is encoded by the CTSL1 gene. The protein is a cysteine cathepsin, a lysosomal cysteine protease that plays a major role in intracellular protein catabolism.
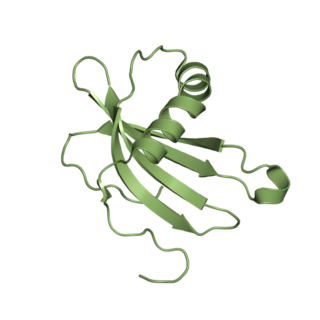
Cystatin-A is a protein that in humans is encoded by the CSTA gene.

Cystatin-B is a protein that in humans is encoded by the CSTB gene.

Cathepsin Z, also called cathepsin X or cathepsin P, is a protein that in humans is encoded by the CTSZ gene. It is a member of the cysteine cathepsin family of cysteine proteases, which has 11 members. As one of the 11 cathepsins, cathepsin Z contains distinctive features from others. Cathepsin Z has been reported involved in cancer malignancy and inflammation.

Cathepsin L2,, is a protein encoded in humans by the CTSV gene.

Cathepsin F is a protein that in humans is encoded by the CTSF gene.
Myeloid and erythroid nuclear termination stage-specific protein (MENT) is a member of the serpin family of protease inhibitors, and participates in DNA and chromatin condensation. Alongside its ability to condense chromatin, MENT is also an effective inhibitor of the proteases cathepsin K, cathepsin L, and cathepsin V, all of which are cysteine proteases. As such, although MENT is structurally classified as a member of the serpin family, it is functionally termed a "cross-class inhibitor," as it is a cysteine rather than a serine protease inhibitor.

Chymopapain is a proteolytic enzyme isolated from the latex of papaya. It is a cysteine protease which belongs to the papain-like protease (PLCP) group. Because of its proteolytic activity, it is the main molecule in the process of chemonucleolysis, used in some procedures like the treatment of herniated lower lumbar discs in the spine by a nonsurgical method.
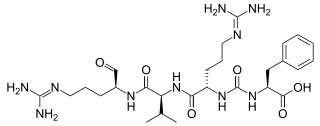
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain. It was discovered in 1972 and was the first natural peptide found that contained an ureylene group. Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases.
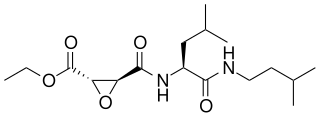
Aloxistatin is a drug which acts as a cysteine protease inhibitor and has anticoagulant effects. It is a synthetic analogue of E-64, a natural product derived from fungi. It was researched for the treatment of muscular dystrophy but was not successful in human clinical trials, though it has continued to be investigated for treatment of spinal cord injury, stroke and Alzheimer's disease. It also shows antiviral effects.

Papain-like proteases are a large protein family of cysteine protease enzymes that share structural and enzymatic properties with the group's namesake member, papain. They are found in all domains of life. In animals, the group is often known as cysteine cathepsins or, in older literature, lysosomal peptidases. In the MEROPS protease enzyme classification system, papain-like proteases form Clan CA. Papain-like proteases share a common catalytic dyad active site featuring a cysteine amino acid residue that acts as a nucleophile.





















