Related Research Articles

Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion.
Autolysins are endogenous lytic enzymes that break down the peptidoglycan components of biological cells which enables the separation of daughter cells following cell division. They are involved in cell growth, cell wall metabolism, cell division and separation, as well as peptidoglycan turnover and have similar functions to lysozymes.

A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.

Staphylococcus aureus is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe that can grow without the need for oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.
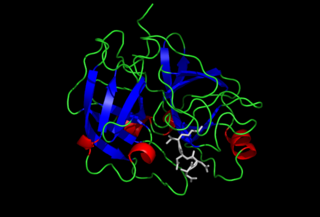
Enteropeptidase is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment.

Staphylococcus epidermidis is a Gram-positive bacterium, and one of over 40 species belonging to the genus Staphylococcus. It is part of the normal human microbiota, typically the skin microbiota, and less commonly the mucosal microbiota and also found in marine sponges. It is a facultative anaerobic bacteria. Although S. epidermidis is not usually pathogenic, patients with compromised immune systems are at risk of developing infection. These infections are generally hospital-acquired. S. epidermidis is a particular concern for people with catheters or other surgical implants because it is known to form biofilms that grow on these devices. Being part of the normal skin microbiota, S. epidermidis is a frequent contaminant of specimens sent to the diagnostic laboratory.

Dihydrolipoamide dehydrogenase (DLD), also known as dihydrolipoyl dehydrogenase, mitochondrial, is an enzyme that in humans is encoded by the DLD gene. DLD is a flavoprotein enzyme that oxidizes dihydrolipoamide to lipoamide.

Ficain also known as ficin, debricin, or higueroxyl delabarre is a proteolytic enzyme extracted from the latex sap from the stems, leaves, and unripe fruit of the American wild fig tree Ficus insipida.

A Disintegrin and metalloproteinase domain-containing protein 10, also known as ADAM10 or CDw156 or CD156c is a protein that in humans is encoded by the ADAM10 gene.
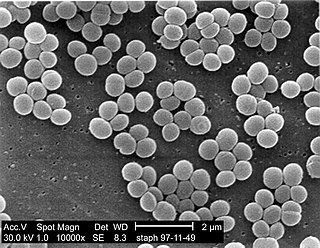
Staphylococcus is a genus of Gram-positive bacteria in the family Staphylococcaceae from the order Bacillales. Under the microscope, they appear spherical (cocci), and form in grape-like clusters. Staphylococcus species are facultative anaerobic organisms.
Sapienic acid is a fatty acid that is a major component of human sebum. Unique to humans, it takes its scientific name from the root sapiens. The equivalent fatty acid in mouse sebum is palmitoleic acid. Sapienic acid salts, esters, anion, and conjugate base are known as sapienates.
Dipeptidyl-peptidase III is an enzyme. This enzyme catalyses the following chemical reaction

Enzybiotics are an experimental antibacterial therapy first described by Nelson, Loomis, and Fischetti. The term is derived from a combination of the words “enzyme” and “antibiotics.” Enzymes have been extensively utilized for their antibacterial and antimicrobial properties. Proteolytic enzymes called endolysins have demonstrated particular effectiveness in combating a range of bacteria and are the basis for enzybiotic research. Endolysins are derived from bacteriophages and are highly efficient at lysing bacterial cells. Enzybiotics are being researched largely to address the issue of antibiotic resistance, which has allowed for the proliferation of drug-resistant pathogens posing great risk to animal and human health across the globe.

Glutamyl endopeptidase is an extracellular bacterial serine protease of the glutamyl endopeptidase I family that was initially isolated from the Staphylococcus aureus strain V8. The protease is, hence, commonly referred to as "V8 protease", or alternatively SspA from its corresponding gene.
C-terminal processing peptidase is an enzyme. This enzyme catalyses the following chemical reaction

Aureolysin is an extracellular metalloprotease expressed by Staphylococcus aureus. This protease is a major contributor to the bacterium's virulence, or ability to cause disease, by cleaving host factors of the innate immune system as well as regulating S. aureus secreted toxins and cell wall proteins. To catalyze its enzymatic activities, aureolysin requires zinc and calcium which it obtains from the extracellular environment within the host.
Snapalysin is an enzyme. This enzyme catalyses the following chemical reaction
Asparagine peptide lyase are one of the seven groups in which proteases, also termed proteolytic enzymes, peptidases, or proteinases, are classified according to their catalytic residue. The catalytic mechanism of the asparagine peptide lyases involves an asparagine residue acting as nucleophile to perform a nucleophilic elimination reaction, rather than hydrolysis, to catalyse the breaking of a peptide bond.
Glutamyl endopeptidase I is a family of extracellular bacterial serine proteases. The proteases within this family have been identified in species of Staphylococcus, Bacillus, and Streptomyces, among others. The two former are more closely related, while the Streptomyces-type is treated as a separate family, glutamyl endopeptidase II.
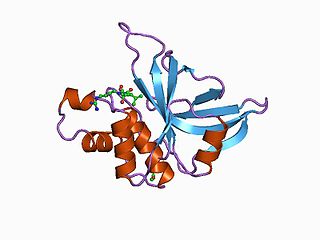
Staphopain A is a secreted cysteine protease produced by Staphylococcus aureus. It was first identified in the S. aureus V8 strain as a papain-like cysteine protease. The protease distinguishes itself from the other major proteases of S. aureus in its very broad specificity and its ability to degrade elastin.
References
- ↑ Hofmann, B.; Hecht, H.J.; Kiess, M.; Schomburg, D. (1993). "Crystal structure of a thiol proteinase from Staphylococcus aureus V8 in the E-64 inhibitor complex". Acta Crystallographica Section A. 49: c102. doi: 10.1107/s0108767378097081 .
- ↑ Potempa J, Dubin A, Travis J (1998). "Staphylopain". In Barrett AJ, Rawlings ND, Woessner JF (eds.). Handbook of Proteolytic Enzymes. London: Handbook of Proteolytic Enzymes. pp. 669–671.
- ↑ Dubin G, Chmiel D, Mak P, Rakwalska M, Rzychon M, Dubin A (November 2001). "Molecular cloning and biochemical characterisation of proteases from Staphylococcus epidermidis". Biological Chemistry. 382 (11): 1575–82. doi:10.1515/bc.2001.192. PMID 11767947.