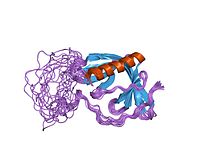
The SRC Homology 3 Domain is a small protein domain of about 60 amino acid residues. Initially, SH3 was described as a conserved sequence in the viral adaptor protein v-Crk. This domain is also present in the molecules of phospholipase and several cytoplasmic tyrosine kinases such as Abl and Src. It has also been identified in several other protein families such as: PI3 Kinase, Ras GTPase-activating protein, CDC24 and cdc25. SH3 domains are found in proteins of signaling pathways regulating the cytoskeleton, the Ras protein, and the Src kinase and many others. The SH3 proteins interact with adaptor proteins and tyrosine kinases. Interacting with tyrosine kinases SH3 proteins usually bind far away from the active site. Approximately 300 SH3 domains are found in proteins encoded in the human genome. In addition to that, the SH3 domain was responsible for controlling protein-protein interactions in the signal transduction pathways and regulating the interactions of proteins involved in the cytoplasmic signaling.

The Wiskott–Aldrich Syndrome protein (WASp) is a 502-amino acid protein expressed in cells of the hematopoietic system that in humans is encoded by the WAS gene. In the inactive state, WASp exists in an autoinhibited conformation with sequences near its C-terminus binding to a region near its N-terminus. Its activation is dependent upon CDC42 and PIP2 acting to disrupt this interaction, causing the WASp protein to 'open'. This exposes a domain near the WASp C-terminus that binds to and activates the Arp2/3 complex. Activated Arp2/3 nucleates new F-actin.

14-3-3 proteins are a family of conserved regulatory molecules that are expressed in all eukaryotic cells. 14-3-3 proteins have the ability to bind a multitude of functionally diverse signaling proteins, including kinases, phosphatases, and transmembrane receptors. More than 200 signaling proteins have been reported as 14-3-3 ligands.
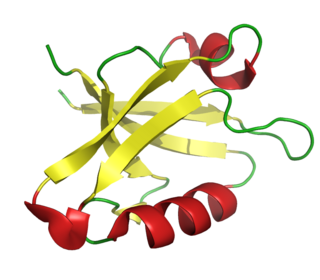
The PDZ domain is a common structural domain of 80-90 amino-acids found in the signaling proteins of bacteria, yeast, plants, viruses and animals. Proteins containing PDZ domains play a key role in anchoring receptor proteins in the membrane to cytoskeletal components. Proteins with these domains help hold together and organize signaling complexes at cellular membranes. These domains play a key role in the formation and function of signal transduction complexes. PDZ domains also play a highly significant role in the anchoring of cell surface receptors to the actin cytoskeleton via mediators like NHERF and ezrin.

The EF hand is a helix-loop-helix structural domain or motif found in a large family of calcium-binding proteins.

Testin also known as TESS is a protein that in humans is encoded by the TES gene located on chromosome 7. TES is a 47 kDa protein composed of 421 amino acids found at focal adhesions and is thought to have a role in regulation of cell motility. In addition to this, TES functions as a tumour suppressor. The TES gene is located within a fragile region of chromosome 7, and the promoter elements of the TES gene have been shown to be susceptible to methylation – this prevents the expression of the TES protein. TES came to greater prominence towards the end of 2007 as a potential mechanism for its tumour suppressor function was published.

ENA/VASP homology proteins or EVH proteins are a family of closely related proteins involved in cell motility in vertebrate and invertebrate animals. EVH proteins are modular proteins that are involved in actin polymerization, as well as interactions with other proteins. Within the cell, Ena/VASP proteins are found at the leading edge of Lamellipodia and at the tips of filopodia. Ena, the founding member of the family was discovered in a drosophila genetic screen for mutations that act as dominant suppressors of the abl non receptor tyrosine kinase. Invertebrate animals have one Ena homologue, whereas mammals have three, named Mena, VASP, and Evl.
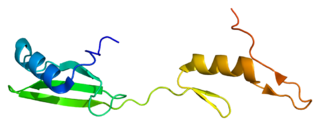
Cytoplasmic protein NCK1 is a protein that in humans is encoded by the NCK1 gene.

Vasodilator-stimulated phosphoprotein is a protein that in humans is encoded by the VASP gene.

Protein enabled homolog is a protein that in humans is encoded by the ENAH gene.
Pre-mRNA-processing factor 40 homolog A is a protein that in humans is encoded by the PRPF40A gene.

WAS/WASL-interacting protein family member 1 (WIP) is a protein that in humans is encoded by the WIPF1 gene.

The WW domain, is a modular protein domain that mediates specific interactions with protein ligands. This domain is found in a number of unrelated signaling and structural proteins and may be repeated up to four times in some proteins. Apart from binding preferentially to proteins that are proline-rich, with particular proline-motifs, [AP]-P-P-[AP]-Y, some WW domains bind to phosphoserine- phosphothreonine-containing motifs.

The Actin assembly-inducing protein (ActA) is a protein encoded and used by Listeria monocytogenes to propel itself through a mammalian host cell. ActA is a bacterial surface protein comprising a membrane-spanning region. In a mammalian cell the bacterial ActA interacts with the Arp2/3 complex and actin monomers to induce actin polymerization on the bacterial surface generating an actin comet tail. The gene encoding ActA is named actA or prtB.

BRCA1 C Terminus (BRCT) domain is a family of evolutionarily related proteins. It is named after the C-terminal domain of BRCA1, a DNA-repair protein that serves as a marker of breast cancer susceptibility.

Formin-like protein 3 (FMNL3), also known as WW domain-binding protein 3 (WBP-3), is a protein that in humans is encoded by the FMNL3 gene.

In structural and cell biology, the focal adhesion targeting domain is a conserved protein domain that was first identified in focal adhesion kinase (FAK), also known as PTK2 protein tyrosine kinase 2 (PTK2).

In molecular biology, the GYF domain is an approximately 60-amino acid protein domain which contains a conserved GP[YF]xxxx[MV]xxWxxx[GN]YF motif. It was identified in the human intracellular protein termed CD2 binding protein 2 (CD2BP2), which binds to a site containing two tandem PPPGHR segments within the cytoplasmic region of CD2. Binding experiments and mutational analyses have demonstrated the critical importance of the GYF tripeptide in ligand binding. A GYF domain is also found in several other eukaryotic proteins of unknown function. It has been proposed that the GYF domain found in these proteins could also be involved in proline-rich sequence recognition. Resolution of the structure of the CD2BP2 GYF domain by NMR spectroscopy revealed a compact domain with a beta-beta-alpha-beta-beta topology, where the single alpha-helix is tilted away from the twisted, anti-parallel beta-sheet. The conserved residues of the GYF domain create a contiguous patch of predominantly hydrophobic nature which forms an integral part of the ligand-binding site. There is limited homology within the C-terminal 20-30 amino acids of various GYF domains, supporting the idea that this part of the domain is structurally but not functionally important.