
Roseola is an infectious disease caused by certain types of virus. Most infections occur before the age of three. Symptoms vary from absent to the classic presentation of a fever of rapid onset followed by a rash. The fever generally lasts for three to five days. The rash is generally pink and lasts for less than three days. Complications may include febrile seizures, with serious complications being rare.

Arbovirus is an informal name used to refer to any viruses that are transmitted by arthropod vectors. The word arbovirus is an acronym. The word tibovirus is sometimes used to more specifically describe viruses transmitted by ticks, a superorder within the arthropods. Arboviruses can affect both animals, including humans, and plants. In humans, symptoms of arbovirus infection generally occur 3–15 days after exposure to the virus and last three or four days. The most common clinical features of infection are fever, headache, and malaise, but encephalitis and hemorrhagic fever may also occur.
Encephalomyelitis is inflammation of the brain and spinal cord. Various types of encephalomyelitis include:
Togaviridae is a family of viruses. Humans, mammals, birds, and mosquitoes serve as natural hosts. There are currently 32 species in this family, divided among 2 genera. Diseases associated with Alphaviruses include arthritis and encephalitis. Rubiviruses such as rubella are sometimes associated with arthritis as well.
Eastern equine encephalitis (EEE), commonly called Triple E or, sleeping sickness is a zoonotic alphavirus and arbovirus present in North, Central, and South America and the Caribbean. EEE was first recognized in Massachusetts, United States, in 1831 when 75 horses died mysteriously of viral encephalitis. Epizootics in horses have continued to occur regularly in the United States. It can also be identified in asses and zebras. Due to the rarity of the disease, its occurrence can cause economic impact in relation to the loss of horses and poultry. EEE is found today in the eastern part of the United States and is often associated with coastal plains. It can most commonly be found in East and Gulf coast states. In Florida, about one to two human cases are reported a year, although over 60 cases of equine encephalitis are reported. Some years in which conditions are favorable for the disease, the number of equine cases is over 200. Diagnosing equine encephalitis is challenging because many of the symptoms are shared with other illnesses and patients can be asymptomatic. Confirmations may require a sample of cerebral spinal fluid or brain tissue, although CT scans and MRI scans are used to detect encephalitis. This could be an indication that the need to test for EEE is necessary. If a biopsy of the cerebral spinal fluid is taken, it is sent to a specialized laboratory for testing.
California encephalitis orthobunyavirus or California encephalitis virus was discovered in Kern County, California and causes encephalitis in humans. Encephalitis is an acute inflammation of the brain that can cause minor symptoms, such as headaches, to more severe symptoms such as seizures. Mosquitoes serve as its carrier and for this reason this virus is known as an arbovirus.
Viral encephalitis is a type of encephalitis caused by a virus.
An emerging infectious disease (EID) is an infectious disease whose incidence has increased in the past 20 years and could increase in the near future. Emerging infections account for at least 12% of all human pathogens. EIDs are caused by newly identified species or strains that may have evolved from a known infection or spread to a new population or to an area undergoing ecologic transformation, or be reemerging infections, like drug resistant tuberculosis. Nosocomial (hospital-acquired) infections, such as methicillin-resistant Staphylococcus aureus are emerging in hospitals, and extremely problematic in that they are resistant to many antibiotics. Of growing concern are adverse synergistic interactions between emerging diseases and other infectious and non-infectious conditions leading to the development of novel syndemics. Many emerging diseases are zoonotic - an animal reservoir incubates the organism, with only occasional transmission into human populations.

Human herpesvirus 6 (HHV-6) is the common collective name for Human betaherpesvirus 6A (HHV-6A) and Human betaherpesvirus 6B (HHV-6B). These closely related viruses are two of the nine herpesviruses known to have humans as their primary host.
Equid alphaherpesvirus 4, formerly Equine herpesvirus 4 (EHV-4) is a virus of the family Herpesviridae that cause rhinopneumonitis in horses. It is the most important viral cause of respiratory infection in foals. Like other herpes viruses, EHV-4 causes a lifelong latent infection in affected animals. These horses are usually the source for new infection for foals over two months old, weanlings, and yearlings. Symptoms include fever, loss of appetite, and discharge from the nose. Most infected animals recover in one to three weeks, but death can occur in environments with overcrowding and other stress factors. There are several vaccines available.
Equid alphaherpesvirus 1, formerly Equine herpesvirus 1 (EHV-1), is a virus of the family Herpesviridae that causes abortion, respiratory disease and occasionally neonatal mortality in horses. Initial spread of EHV-1 by a newly introduced horse through direct and indirect contact can lead to abortion and perinatal infection in up to 70 percent of a previously unexposed herd. Abortion usually occurs in the last four months of gestation, two to four weeks after infection of the mare. Perinatal infection can lead to pneumonia and death. Encephalitis can occur in affected animals, leading to ataxia, paralysis, and death. There is a vaccine available, however its efficacy is questionable.The virus varies in severity from sub-clinical to very severe. Most horses have been infected with EHV-1 but the virus can become latent and show no signs and never be an issue. In 2006, an outbreak of EHV-1 among stables in Florida resulted in the institution of various quarantine measures. The outbreak was determined to have originated with several horses imported from Europe via New York, and then shipped to Florida.
Equid gammaherpesvirus 2, formerly Equine herpesvirus 2 (EHV-2), is a virus of the family Herpesviridae, originally known as equine cytomegalovirus due to its slow replication in tissue culture. However, complete sequencing of the EHV-2 genome has demonstrated that it is a member of the subfamily Gammaherpesvirinae, in the genus Percavirus. It has an uncertain role in respiratory disease in horses, but EHV-2 has been isolated from cases exhibiting symptoms such as coughing, conjunctivitis, and swollen submaxillary and parotid lymph nodes.

Venezuelan equine encephalitis virus is a mosquito-borne viral pathogen that causes Venezuelan equine encephalitis or encephalomyelitis (VEE). VEE can affect all equine species, such as horses, donkeys, and zebras. After infection, equines may suddenly die or show progressive central nervous system disorders. Humans also can contract this disease. Healthy adults who become infected by the virus may experience flu-like symptoms, such as high fevers and headaches. People with weakened immune systems and the young and the elderly can become severely ill or die from this disease.
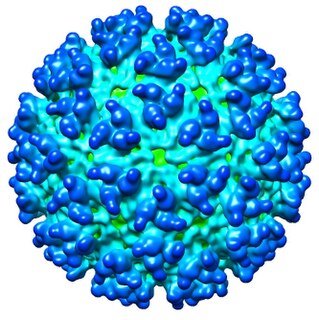
The Western equine encephalomyelitis virus is the causative agent of relatively uncommon viral disease Western equine encephalomyelitis (WEE). An Alphavirus of the family Togaviridae, the WEE virus is an arbovirus transmitted by mosquitoes of the genera Culex and Culiseta. WEE is a recombinant virus between two other alphaviruses, an ancestral Sindbis virus-like virus, and an ancestral Eastern equine encephalitis virus-like virus. There have been under 700 confirmed cases in the U.S. since 1964. This virus contains an envelope that is made up of glycoproteins and nucleic acids. The virus is transmitted to people and horses by bites from infected mosquitoes and birds during wet, summer months.

Cyclic AMP-responsive element-binding protein 3 is a protein that in humans is encoded by the CREB3 gene.
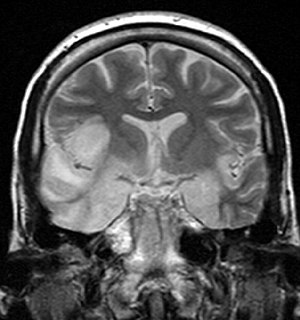
Herpesviral encephalitis is encephalitis due to herpes simplex virus.

Banna virus (BAV) is a virus belonging to Reoviridae, a family of segmented, non-enveloped, double-stranded RNA viruses. It is an arbovirus, being primarily transmitted to humans from the bite of infected mosquitoes of the genus Culex. Pigs and cattle have also been shown to become infected. The most common symptom of infection is fever, but in some cases encephalitis may occur. There is no specific treatment for infection, so treatment is aimed at alleviating the severity of symptoms until the immune system has cleared the infection.
Simone Warner is an Australian scientist, a microbiology researcher. She currently leads the Microbiology group within the Victorian Department of Environment and Primary Industries, based at the Centre for AgriBiosciences in Bundoora, situated on the La Trobe University campus.
Jamestown Canyon encephalitis is an infectious disease caused by the Jamestown Canyon virus, an orthobunyavirus of the California serogroup. It is mainly spread during the summer by different mosquito species in the United States and Canada.










