
N-Methyl-D-aspartic acid or N-Methyl-D-aspartate (NMDA) is an amino acid derivative that acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor. Unlike glutamate, NMDA only binds to and regulates the NMDA receptor and has no effect on other glutamate receptors. NMDA receptors are particularly important when they become overactive during, for example, withdrawal from alcohol as this causes symptoms such as agitation and, sometimes, epileptiform seizures.
A biogenic amine is a biogenic substance with one or more amine groups. They are basic nitrogenous compounds formed mainly by decarboxylation of amino acids or by amination and transamination of aldehydes and ketones. Biogenic amines are organic bases with low molecular weight and are synthesized by microbial, vegetable and animal metabolisms. In food and beverages they are formed by the enzymes of raw material or are generated by microbial decarboxylation of amino acids.
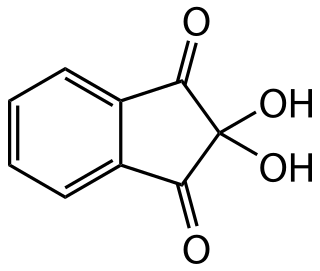
Ninhydrin (2,2-dihydroxyindane-1,3-dione) is a chemical used to detect ammonia or primary and secondary amines. When reacting with these free amines, a deep blue or purple color known as Ruhemann's purple is produced. Ninhydrin is most commonly used to detect fingerprints, as the terminal amines of lysine residues in peptides and proteins sloughed off in fingerprints react with ninhydrin. It is a white solid which is soluble in ethanol and acetone at room temperature. Ninhydrin can be considered as the hydrate of indane-1,2,3-trione.

Gas chromatography–mass spectrometry (GC-MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC-MS include drug detection, fire investigation, environmental analysis, explosives investigation, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC-MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.
Protein sequencing is the practical process of determining the amino acid sequence of all or part of a protein or peptide. This may serve to identify the protein or characterize its post-translational modifications. Typically, partial sequencing of a protein provides sufficient information to identify it with reference to databases of protein sequences derived from the conceptual translation of genes.
Inborn errors of metabolism form a large class of genetic diseases involving congenital disorders of enzyme activities. The majority are due to defects of single genes that code for enzymes that facilitate conversion of various substances (substrates) into others (products). In most of the disorders, problems arise due to accumulation of substances which are toxic or interfere with normal function, or due to the effects of reduced ability to synthesize essential compounds. Inborn errors of metabolism are now often referred to as congenital metabolic diseases or inherited metabolic disorders. To this concept it's possible to include the new term of Enzymopathy. This term was created following the study of Biodynamic Enzymology, a science based on the study of the enzymes and their derivated products. Finally, inborn errors of metabolism were studied for the first time by British physician Archibald Garrod (1857–1936), in 1908. He is known for work that prefigured the "one gene-one enzyme" hypothesis, based on his studies on the nature and inheritance of alkaptonuria. His seminal text, Inborn Errors of Metabolism, was published in 1923.

Ion chromatography separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one unit away from the isoelectric point of a protein.
Protein methods are the techniques used to study proteins. There are experimental methods for studying proteins. Computational methods typically use computer programs to analyze proteins. However, many experimental methods require computational analysis of the raw data.

The biurettest, also known as Piotrowski's test, is a chemical test used for detecting the presence of peptide bonds. In the presence of peptides, a copper(II) ion forms mauve-colored coordination complexes in an alkaline solution. Several variants on the test have been developed, such as the BCA test and the Modified Lowry test.

A chiral derivatizing agent (CDA) also known as a chiral resolving reagent, is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present within the mix. Analysis can be conducted by spectroscopy or by chromatography. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.
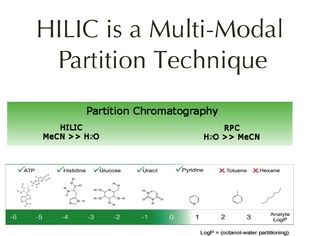
Hydrophilic interaction chromatography is a variant of normal phase liquid chromatography that partly overlaps with other chromatographic applications such as ion chromatography and reversed phase liquid chromatography. HILIC uses hydrophilic stationary phases with reversed-phase type eluents. The name was suggested by Andrew Alpert in his 1990 paper on the subject. He described the chromatographic mechanism for it as liquid-liquid partition chromatography where analytes elute in order of increasing polarity, a conclusion supported by a review and re-evaluation of published data.
Micellar liquid chromatography (MLC) is a form of reversed phase liquid chromatography that uses an aqueous micellar solutions as the mobile phase.

Lawsone (2-hydroxy-1,4-naphthoquinone), also known as hennotannic acid, is a red-orange dye present in the leaves of the henna plant, for which it is named, as well as in the flower of water hyacinth. Humans have used henna extracts containing lawsone as hair and skin dyes for more than 5000 years. Lawsone reacts chemically with the protein keratin in skin and hair via Michael addition, resulting in a strong permanent stain that lasts until the skin or hair is shed. The darker colored ink is due to more lawsone-keratin interactions occurring, which evidently break down as the concentration of lawsone decreases and the tattoo fades. Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning and sunscreens. Chemically, lawsone is a 1,4-naphthoquinone derivative.

The Folin–Ciocâlteu reagent (FCR) or Folin's phenol reagent or Folin–Denis reagent, also called the gallic acid equivalence method (GAE), is a mixture of phosphomolybdate and phosphotungstate used for the colorimetric in vitro assay of phenolic and polyphenolic antioxidants. It is named after Otto Folin, Vintilă Ciocâlteu, and Willey Glover Denis.

Pyrolysis–gas chromatography–mass spectrometry is a method of chemical analysis in which the sample is heated to decomposition to produce smaller molecules that are separated by gas chromatography and detected using mass spectrometry.

1-Fluoro-2,4-dinitrobenzene is a chemical that reacts with the N-terminal amino acid of polypeptides. This can be helpful for sequencing proteins.

p-Dimethylaminocinnamaldehyde (DMACA) is an aromatic hydrocarbon. It is used in an acidic solution to detect indoles.
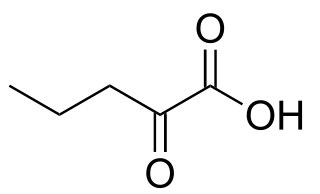
α-Ketovaleric acid is a keto acid that is found in human blood. Unlike related keto acids, it is not an intermediate or metabolite associated with amino acids and its origin is unknown.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.
















