Acyl-CoA dehydrogenases (ACADs) are a class of enzymes that function to catalyze the initial step in each cycle of fatty acid β-oxidation in the mitochondria of cells. Their action results in the introduction of a trans double-bond between C2 (α) and C3 (β) of the acyl-CoA thioester substrate. Flavin adenine dinucleotide (FAD) is a required co-factor in addition to the presence of an active site glutamate in order for the enzyme to function.
Oxalobacter formigenes is a Gram negative oxalate-degrading anaerobic bacterium that was first isolated from the gastrointestinal tract of a sheep in 1985. To date, the bacterium has been found to colonize the large intestines of numerous vertebrates, including humans, and has even been isolated from freshwater sediment. It processes oxalate by decarboxylation into formate, producing energy for itself in the process.
In enzymology, a precorrin-4 C11-methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-8X methylmutase is an enzyme that catalyzes the chemical reaction
In enzymology, an acetate CoA-transferase is an enzyme that catalyzes the chemical reaction
In enzymology, an oxalate CoA-transferase is an enzyme that catalyzes the chemical reaction
In enzymology, a succinyl-CoA:(R)-benzylsuccinate CoA-transferase is an enzyme that catalyzes the chemical reaction
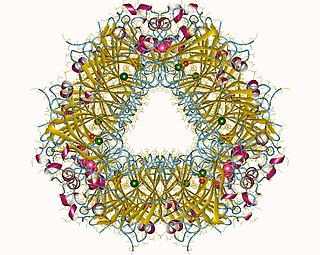
In enzymology, an oxalate decarboxylase (EC 4.1.1.2) is an oxalate degrading enzyme that catalyzes the chemical reaction
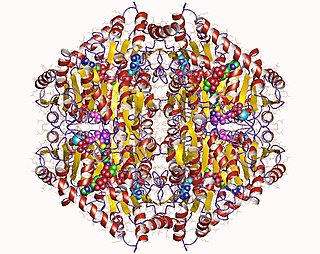
The enzyme oxalyl-CoA decarboxylase (OXC) (EC 4.1.1.8), primarily produced by the gastrointestinal bacterium Oxalobacter formigenes, catalyzes the chemical reaction
In enzymology, an oxalate—CoA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-oxoadipyl-CoA thiolase is an enzyme that catalyzes the chemical reaction
In enzymology, an arginine N-succinyltransferase (EC 2.3.1.109) is an enzyme that catalyzes the chemical reaction
In enzymology, formate C-acetyltransferase is an enzyme. Pyruvate formate lyase is found in Escherichia coli and other organisms. It helps regulate anaerobic glucose metabolism. Using radical non-redox chemistry, it catalyzes the reversible conversion of pyruvate and coenzyme-A into formate and acetyl-CoA. The reaction occurs as follows:
In enzymology, a diaminobutyrate-2-oxoglutarate transaminase is an enzyme that catalyzes the chemical reaction

In enzymology, a succinylornithine transaminase (EC 2.6.1.81) is an enzyme that catalyzes the chemical reaction

In molecular biology, cob(I)yrinic acid a,c-diamide adenosyltransferase EC 2.5.1.17 is an enzyme which catalyses the conversion of cobalamin into one of its coenzyme forms, adenosylcobalamin. Adenosylcobalamin is required as a cofactor for the activity of certain enzymes. AdoCbl contains an adenosyl moiety liganded to the cobalt ion of cobalamin via a covalent Co-C bond.

An oxalate degrading enzyme is a type of enzyme that catalyzes the biodegradation of oxalate. Enzymes in this class include oxalate oxidase, oxalate decarboxylase, oxalyl-CoA decarboxylase, and formyl-CoA transferase.
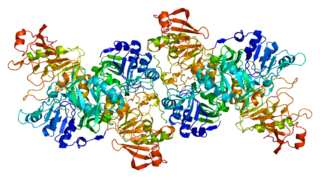
Coenzyme A transferases (CoA-transferases) are transferase enzymes that catalyze the transfer of a coenzyme A group from an acyl-CoA donor to a carboxylic acid acceptor. Among other roles, they are responsible for transfer of CoA groups during fermentation and metabolism of ketone bodies. These enzymes are found in all three domains of life.
Oxalobacter aliiformigenes is a Gram negative, non-spore-forming, oxalate-degrading anaerobic bacterium that was first isolated from human fecal samples. O. aliiformigenes consumes oxalate as its main carbon source but is negative for indole production and negative for sulfate and nitrate reduction. Cells appear rod shaped, though occasionally present as curved, and do not possess flagella.
Oxalobacter paraformigenes is a Gram negative, non-spore-forming, oxalate-degrading anaerobic bacterium that was first isolated from human fecal samples. O. paraformigenes may have a role in calcium oxalate kidney stone disease because of its unique ability to utilize oxalate as its primary carbon source.







