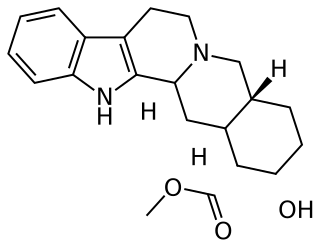
Yohimbine, also known as quebrachine, is an indole alkaloid derived from the bark of the African tree Pausinystalia johimbe; also from the bark of the unrelated South American tree Aspidosperma quebracho-blanco. Yohimbine is an α2-adrenergic receptor antagonist, and has been used in a variety of research projects. It is a veterinary drug used to reverse sedation in dogs and deer.

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.
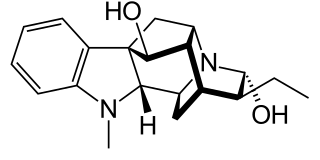
Ajmaline is an alkaloid that is classified as a 1-A antiarrhythmic agent. It is often used to induce arrhythmic contraction in patients suspected of having Brugada syndrome. Individuals suffering from Brugada syndrome will be more susceptible to the arrhythmogenic effects of the drug, and this can be observed on an electrocardiogram as an ST elevation.

Alstonia scholaris, commonly called blackboard tree, scholar tree, milkwood or devil's tree in English, is an evergreen tropical tree in the Dogbane Family (Apocynaceae). It is native to southern China, tropical Asia and Australasia, where it is a common ornamental plant. It is a toxic plant, but is used traditionally for myriad diseases and complaints.

Yuremamine is a phytoindole alkaloid which was isolated from the bark of Mimosa tenuiflora in 2005, and erroneously assigned a pyrrolo[1,2-a]indole structure that was thought to represent a new class of indole alkaloids. However, in 2015, the bioinspired total synthesis of yuremamine revealed its structure to be a flavonoid derivative. It was also noted in the original isolation of yuremamine that the alkaloid occurs naturally as a purple solid, but total synthesis revealed that yuremamine as a free base is colorless, and the formation of a trifluoroacetate salt during HPLC purification is what led to the purple appearance.

Tetrahydroharman(e), also known as 1-methyl-1,2,3,4-tetrahydro-β-carboline, is a general name for one of two isomers:
- (1S)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole
- Calligonine ((1R)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole)

Pericine is one of a number of indole alkaloids found in the tree Picralima nitida, commonly known as akuamma. As with some other alkaloids from this plant such as akuammine, pericine has been shown to bind to mu opioid receptors in vitro, and has an IC50 of 0.6 μmol, within the range of a weak analgesic. It may also have convulsant effects.

Conessine is a steroidal alkaloid found in a number of plant species from the family Apocynaceae, including Holarrhena floribunda, Holarrhena antidysenterica and Funtumia elastica. It acts as a histamine antagonist, selective for the H3 subtype (with an affinity of pKi = 8.27; Ki = ~5 nM). It was also found to have long CNS clearance times, high blood–brain barrier penetration and high affinity for the adrenergic receptors.

Ziziphus oenopolia, commonly known as the jackal jujube, small-fruited jujube or wild jujube, is a flowering plant with a broad distribution through tropical and subtropical Asia and Australasia. In India, it is mostly found in the deciduous forests of the southern part of the country.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Affinine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally it can be considered a member of the vobasine alkaloid family and may be synthesized from tryptophan. Limited pharmacological testing has indicated that it may be an effective inhibitor of both acetylcholinesterase and butyrylcholinesterase.
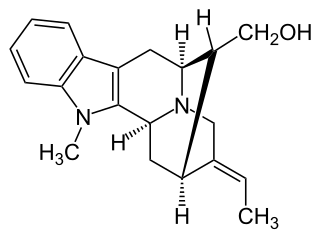
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Dregamine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Ervatamia hirta and Tabernaemontana divaricata.

Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Voacristine is a indole alkaloid occurring in Voacanga and Tabernaemontana genus. It is also an iboga type alkaloid.
Strychnos icaja is a species belonging to the plant family Loganiaceae, native to West Tropical Africa. It is a very large, tropical rainforest liana which may attain a length of 100 m (330 ft).


















