
A bacteriophage, also known informally as a phage, is a virus that infects and replicates within bacteria and archaea. The term is derived from Ancient Greek φαγεῖν (phagein) 'to devour' and bacteria. Bacteriophages are composed of proteins that encapsulate a DNA or RNA genome, and may have structures that are either simple or elaborate. Their genomes may encode as few as four genes and as many as hundreds of genes. Phages replicate within the bacterium following the injection of their genome into its cytoplasm.
Molecular biology is a branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions.
This timeline of biology and organic chemistry captures significant events from before 1600 to the present.

Salvador Edward Luria was an Italian microbiologist, later a naturalized U.S. citizen. He won the Nobel Prize in Physiology or Medicine in 1969, with Max Delbrück and Alfred Hershey, for their discoveries on the replication mechanism and the genetic structure of viruses. Salvador Luria also showed that bacterial resistance to viruses (phages) is genetically inherited.

Martha Cowles Chase, also known as Martha C. Epstein, was an American geneticist who in 1952, with Alfred Hershey, experimentally helped to confirm that DNA rather than protein is the genetic material of life.

Enterobacteria phage T2 is a virus that infects and kills E. coli. It is in the genus Tequatrovirus, and the family Myoviridae. Its genome consists of linear double-stranded DNA, with repeats at either end. The phage is covered by a protective protein coat.

The lytic cycle is one of the two cycles of viral reproduction, the other being the lysogenic cycle. The lytic cycle results in the destruction of the infected cell and its membrane. Bacteriophages that can only go through the lytic cycle are called virulent phages.

Escherichia virus T4 is a species of bacteriophages that infect Escherichia coli bacteria. It is a double-stranded DNA virus in the subfamily Tevenvirinae of the family Straboviridae. T4 is capable of undergoing only a lytic life cycle and not the lysogenic life cycle. The species was formerly named T-even bacteriophage, a name which also encompasses, among other strains, Enterobacteria phage T2, Enterobacteria phage T4 and Enterobacteria phage T6.

Alfred Day Hershey was an American Nobel Prize–winning bacteriologist and geneticist.

Filamentous bacteriophages are a family of viruses (Inoviridae) that infect bacteria, or bacteriophages. They are named for their filamentous shape, a worm-like chain, about 6 nm in diameter and about 1000-2000 nm long. This distinctive shape reflects their method of replication: the coat of the virion comprises five types of viral protein, which are located in the inner membrane of the host bacterium during phage assembly, and these proteins are added to the nascent virion's DNA as it is extruded through the membrane. The simplicity of filamentous phages makes them an appealing model organism for research in molecular biology, and they have also shown promise as tools in nanotechnology and immunology.

The history of genetics dates from the classical era with contributions by Pythagoras, Hippocrates, Aristotle, Epicurus, and others. Modern genetics began with the work of the Augustinian friar Gregor Johann Mendel. His works on pea plants, published in 1866, provided the initial evidence that, on its rediscovery in 1900's, helped to establish the theory of Mendelian inheritance.

M13 is one of the Ff phages, a member of the family filamentous bacteriophage (inovirus). Ff phages are composed of circular single-stranded DNA (ssDNA), which in the case of the m13 phage is 6407 nucleotides long and is encapsulated in approximately 2700 copies of the major coat protein p8, and capped with about 5 copies each of four different minor coat proteins. The minor coat protein p3 attaches to the receptor at the tip of the F pilus of the host Escherichia coli. The life cycle is relatively short, with the early phage progeny exiting the cell ten minutes after infection. Ff phages are chronic phage, releasing their progeny without killing the host cells. The infection causes turbid plaques in E. coli lawns, of intermediate opacity in comparison to regular lysis plaques. However, a decrease in the rate of cell growth is seen in the infected cells. The replicative form of M13 is circular double-stranded DNA similar to plasmids that are used for many recombinant DNA processes, and the virus has also been used for phage display, directed evolution, nanostructures and nanotechnology applications.

Lysogeny, or the lysogenic cycle, is one of two cycles of viral reproduction. Lysogeny is characterized by integration of the bacteriophage nucleic acid into the host bacterium's genome or formation of a circular replicon in the bacterial cytoplasm. In this condition the bacterium continues to live and reproduce normally, while the bacteriophage lies in a dormant state in the host cell. The genetic material of the bacteriophage, called a prophage, can be transmitted to daughter cells at each subsequent cell division, and later events can release it, causing proliferation of new phages via the lytic cycle.
The history of molecular biology begins in the 1930s with the convergence of various, previously distinct biological and physical disciplines: biochemistry, genetics, microbiology, virology and physics. With the hope of understanding life at its most fundamental level, numerous physicists and chemists also took an interest in what would become molecular biology.
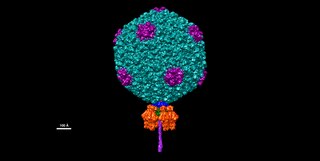
Salmonella virus P22 is a bacteriophage in the Podoviridae family that infects Salmonella typhimurium. Like many phages, it has been used in molecular biology to induce mutations in cultured bacteria and to introduce foreign genetic material. P22 has been used in generalized transduction and is an important tool for investigating Salmonella genetics.
The phage group was an informal network of biologists centered on Max Delbrück that contributed heavily to bacterial genetics and the origins of molecular biology in the mid-20th century. The phage group takes its name from bacteriophages, the bacteria-infecting viruses that the group used as experimental model organisms. In addition to Delbrück, important scientists associated with the phage group include: Salvador Luria, Alfred Hershey, Seymour Benzer, Charles Steinberg, Gunther Stent, James D. Watson, Frank Stahl, and Renato Dulbecco.

The Avery–MacLeod–McCarty experiment was an experimental demonstration by Oswald Avery, Colin MacLeod, and Maclyn McCarty that, in 1944, reported that DNA is the substance that causes bacterial transformation, in an era when it had been widely believed that it was proteins that served the function of carrying genetic information. It was the culmination of research in the 1930s and early 20th century at the Rockefeller Institute for Medical Research to purify and characterize the "transforming principle" responsible for the transformation phenomenon first described in Griffith's experiment of 1928: killed Streptococcus pneumoniae of the virulent strain type III-S, when injected along with living but non-virulent type II-R pneumococci, resulted in a deadly infection of type III-S pneumococci. In their paper "Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type III", published in the February 1944 issue of the Journal of Experimental Medicine, Avery and his colleagues suggest that DNA, rather than protein as widely believed at the time, may be the hereditary material of bacteria, and could be analogous to genes and/or viruses in higher organisms.

A corynebacteriophage is a DNA-containing bacteriophage specific for bacteria of genus Corynebacterium as its host. Corynebacterium diphtheriae virus strain Corynebacterium diphtheriae phage introduces toxigenicity into strains of Corynebacterium diphtheriae as it encodes diphtheria toxin, it has subtypes beta c and beta vir. According to proposed taxonomic classification, corynephages β and ω are unclassified members of the genus Lambdavirus, family Siphoviridae.
The history of genetics can be represented on a timeline of events from the earliest work in the 1850s, to the DNA era starting in the 1940s, and the genomics era beginning in the 1970s.

Ff phages is a group of almost identical filamentous phage including phages f1, fd, M13 and ZJ/2, which infect bacteria bearing the F fertility factor. The virion is a flexible filament measuring about 6 by 900 nm, comprising a cylindrical protein tube protecting a single-stranded circular DNA molecule at its core. The phage codes for only 11 gene products, and is one of the simplest viruses known. It has been widely used to study fundamental aspects of molecular biology. George Smith and Greg Winter used f1 and fd for their work on phage display for which they were awarded a share of the 2018 Nobel Prize in Chemistry. Early experiments on Ff phages used M13 to identify gene functions, and M13 was also developed as a cloning vehicle, so the name M13 is sometimes used as an informal synonym for the whole group of Ff phages.















