Related Research Articles
Endorphins are peptides produced in the brain that block the perception of pain and increase feelings of wellbeing. They are produced and stored in the pituitary gland of the brain. Endorphins are endogenous painkillers often produced in the brain and adrenal medulla during physical exercise or orgasm and inhibit pain, muscle cramps, and relieve stress.
Diabetic neuropathy is various types of nerve damage associated with diabetes mellitus. Symptoms depend on the site of nerve damage and can include motor changes such as weakness; sensory symptoms such as numbness, tingling, or pain; or autonomic changes such as urinary symptoms. These changes are thought to result from a microvascular injury involving small blood vessels that supply nerves. Relatively common conditions which may be associated with diabetic neuropathy include distal symmetric polyneuropathy; third, fourth, or sixth cranial nerve palsy; mononeuropathy; mononeuropathy multiplex; diabetic amyotrophy; and autonomic neuropathy.
Congenital insensitivity to pain (CIP), also known as congenital analgesia, is one or more extraordinarily rare conditions in which a person cannot feel physical pain. The conditions described here are separate from the HSAN group of disorders, which have more specific signs and cause. Because feeling physical pain is vital for survival, CIP is an extremely dangerous condition. It is common for people with the condition to die in childhood due to injuries or illnesses going unnoticed. Burn injuries are among the more common injuries.
Dynorphins (Dyn) are a class of opioid peptides that arise from the precursor protein prodynorphin. When prodynorphin is cleaved during processing by proprotein convertase 2 (PC2), multiple active peptides are released: dynorphin A, dynorphin B, and α/β-neoendorphin. Depolarization of a neuron containing prodynorphin stimulates PC2 processing, which occurs within synaptic vesicles in the presynaptic terminal. Occasionally, prodynorphin is not fully processed, leading to the release of “big dynorphin.” “Big Dynorphin” is a 32-amino acid molecule consisting of both dynorphin A and dynorphin B.
Neuropathic pain is pain caused by a lesion or disease of the somatosensory nervous system. Neuropathic pain may be associated with abnormal sensations called dysesthesia or pain from normally non-painful stimuli (allodynia). It may have continuous and/or episodic (paroxysmal) components. The latter resemble stabbings or electric shocks. Common qualities include burning or coldness, "pins and needles" sensations, numbness and itching.
Some philosophers, such as Jeremy Bentham, Baruch Spinoza, and Descartes, have hypothesized that the feelings of pain and pleasure are part of a continuum.

Nociceptin/orphanin FQ (N/OFQ), a 17-amino acid neuropeptide, is the endogenous ligand for the nociceptin receptor. Nociceptin acts as a potent anti-analgesic, effectively counteracting the effect of pain-relievers; its activation is associated with brain functions such as pain sensation and fear learning.
Opioid-induced hyperalgesia (OIH) or opioid-induced abnormal pain sensitivity, also called paradoxical hyperalgesia, is an uncommon condition of generalized pain caused by the long-term use of high dosages of opioids such as morphine, oxycodone, and methadone. OIH is not necessarily confined to the original affected site. This means that if the person was originally taking opioids due to lower back pain, when OIH appears, the person may experience pain in the entire body, instead of just in the lower back. Over time, individuals taking opioids can also develop an increasing sensitivity to noxious stimuli, even evolving a painful response to previously non-noxious stimuli (allodynia). This means that if the person originally felt pain from twisting or from sitting too long, the person might now additionally experience pain from a light touch or from raindrops falling on the skin.
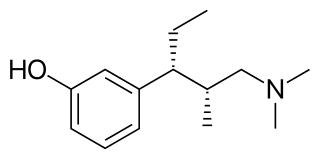
Tapentadol, brand names Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analgesia occurs within 32 minutes of oral administration, and lasts for 4–6 hours.

Proglumide (Milid) is a drug that inhibits gastrointestinal motility and reduces gastric secretions. It acts as a cholecystokinin antagonist, which blocks both the CCKA and CCKB subtypes. It was used mainly in the treatment of stomach ulcers, although it has now been largely replaced by newer drugs for this application.
Procedural sedation and analgesia (PSA) is a technique in which a sedating/dissociative medication is given, usually along with an analgesic medication, in order to perform non-surgical procedures on a patient. The overall goal is to induce a decreased level of consciousness while maintaining the patient's ability to breathe on their own. Airway protective reflexes are not compromised by this process and therefore endotracheal intubation is not required. PSA is commonly used in the emergency department, in addition to the operating room.
Electroanalgesia is a form of analgesia, or pain relief, that uses electricity to ease pain. Electrical devices can be internal or external, at the site of pain (local) or delocalized throughout the whole body. It works by interfering with the electric currents of pain signals, inhibiting them from reaching the brain and inducing a response; different from traditional analgesics, such as opiates which mimic natural endorphins and NSAIDs that help relieve inflammation and stop pain at the source. Electroanalgesia has a lower addictive potential and poses less health threats to the general public, but can cause serious health problems, even death, in people with other electrical devices such as pacemakers or internal hearing aids, or with heart problems.

There is a scientific debate which questions whether crustaceans experience pain. It is a complex mental state, with a distinct perceptual quality but also associated with suffering, which is an emotional state. Because of this complexity, the presence of pain in an animal, or another human for that matter, cannot be determined unambiguously using observational methods, but the conclusion that animals experience pain is often inferred on the basis of likely presence of phenomenal consciousness which is deduced from comparative brain physiology as well as physical and behavioural reactions.
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, and lipid modulator PEA has been studied in in vitro and in vivo systems using exogenously added or dosed compound; there is evidence that it binds to a nuclear receptor, through which it exerts a variety of biological effects, some related to chronic inflammation and pain.

Clinical neurochemistry is the field of neurological biochemistry which relates biochemical phenomena to clinical symptomatic manifestations in humans. While neurochemistry is mostly associated with the effects of neurotransmitters and similarly functioning chemicals on neurons themselves, clinical neurochemistry relates these phenomena to system-wide symptoms. Clinical neurochemistry is related to neurogenesis, neuromodulation, neuroplasticity, neuroendocrinology, and neuroimmunology in the context of associating neurological findings at both lower and higher level organismal functions.
Pain in cancer may arise from a tumor compressing or infiltrating nearby body parts; from treatments and diagnostic procedures; or from skin, nerve and other changes caused by a hormone imbalance or immune response. Most chronic (long-lasting) pain is caused by the illness and most acute (short-term) pain is caused by treatment or diagnostic procedures. However, radiotherapy, surgery and chemotherapy may produce painful conditions that persist long after treatment has ended.
Gaseous signaling molecules are gaseous molecules that are either synthesized internally (endogenously) in the organism, tissue or cell or are received by the organism, tissue or cell from outside and that are used to transmit chemical signals which induce certain physiological or biochemical changes in the organism, tissue or cell. The term is applied to, for example, oxygen, carbon dioxide, sulfur dioxide, nitrous oxide, hydrogen cyanide, ammonia, methane, hydrogen, ethylene, etc.

RB-120 is an orally active analog of the drug RB-101. It acts as an enkephalinase inhibitor, which is used in scientific research. Via intravenous administration, it is approximately three times as potent as RB-101 or twice as potent as the isolated (S,S) isomer of RB101. However, via i.p. administration it is approximately twice as potent as racemic RB-101 and about as potent as the isolated (S,S) isomer of RB101. During i.v. administration RB120 is approximately twice as weak as morphine in terms of analgesia; however, it is 16x weaker during i.p. and p.o. administration.
Peripheral mononeuropathy is a nerve related disease where a single nerve, that is used to transport messages from the brain to the peripheral body, is diseased or damaged. Peripheral neuropathy is a general term that indicates any disorder of the peripheral nervous system. The name of the disorder itself can be broken down in order to understand this better; peripheral: in regard to peripheral neuropathy, refers to outside of the brain and spinal cord; neuro: means nerve related; -pathy; means disease. Peripheral mononeuropathy is a disorder that links to Peripheral Neuropathy, as it only effects a single peripheral nerve rather than several damaged or diseased nerves throughout the body. Healthy peripheral nerves are able to “carry messages from the brain and spinal cord to muscles, organs, and other body tissues”.
Placebo analgesia occurs when the administration of placebos leads to pain relief. Because placebos by definition lack active ingredients, the effect of placebo analgesia is considered to result from the patient's belief that they are receiving an analgesic drug or other medical intervention. It has been shown that, in some cases, the endogenous opioid system is critical for mediating placebo analgesia, as evidenced by the ability of such analgesia to be reduced by the opioid antagonist naloxone. However, it is also possible for placebo analgesia to be mediated by non-opioid mechanisms, in which case it would not be affected by naloxone. Other research has indicated that the human spinal cord, prefrontal cortex, and rostral anterior cingulate cortex also play a role in placebo analgesia.
References
- ↑ Vane, J. (2003). "The mechanism of action of anti-inflammatory drugs." Int J Clin Pract Suppl (135): 2.
- ↑ Yang, J., Y. Yang, et al. (2007). "Effect of oxytocin on acupuncture analgesia in the rat." Neuropeptides 41(5): 285–92.
- ↑ Koltyn, K. F. and M. Umeda (2006). "Exercise, hypoalgesia and blood pressure." Sports Med 36(3): 207–14.
- ↑ Koltyn, K. F. (2000). "Analgesia following exercise: a review." Sports Med 29(2): 85–98.
- ↑ Fuss, J., Steinle, J., Bindila, L., Auer, M.K., Kirchherr, H., Lutz, B., Gass. P. (2015). "A runner's high depends on cannabinoid receptors in mice." Proc Natl Acad Sci 112(42): 13105–08.
- ↑ Rhudy, J. L., J. S. Grimes, et al. (2004). "Fear-induced hypoalgesia in humans: effects on low intensity thermal stimulation and finger temperature." J Pain 5(8): 458–68.
- ↑ J.M. Lichtman and M.S. Fanselow, "Cats produce analgesia in rats on the tail-flick test: naltrexone sensitivity is determined by the nociceptive test stimulus". Brain Res 533 (1990), pp. 91–94.
- ↑ H.S. Hagen and K.F. Green, "Effects of time of testing, stress level and number of conditioning days on naloxone sensitivity of conditioned stress-induced analgesia in rats". Behav Neurosci 102 (1988), pp. 906–14.
- ↑ Schalka, M. M., M. S. Correa, et al. (2006). "Congenital insensitivity-to-pain with anhidrosis (CIPA): a case report with 4-year follow-up." Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(6): 769–73.
- ↑ Zamir, N., Shuber, E., 1980. "Altered pain perception in hypertensive humans". Brain Research 201, 471–74.
- ↑ Edwards, L., C. Ring, et al. (2007). "Nociceptive flexion reflex thresholds and pain during rest and computer game play in patients with hypertension and individuals at risk for hypertension." Biol Psychol 76(1-2): 72–82.