Related Research Articles

Protein folding is the physical process by which a protein, after synthesis by a ribosome as a linear chain of amino acids, changes from an unstable random coil into a more ordered three-dimensional structure. This structure permits the protein to become biologically functional.

Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure and ability to be stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal structure and physiological functions (misfolding) and form fibrous deposits within and around cells. These protein misfolding and deposition processes disrupt the healthy function of tissues and organs.
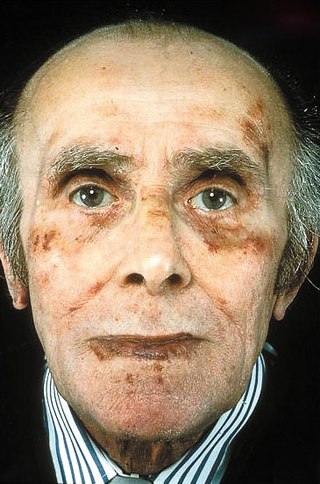
Amyloidosis is a group of diseases in which abnormal proteins, known as amyloid fibrils, build up in tissue. There are several non-specific and vague signs and symptoms associated with amyloidosis. These include fatigue, peripheral edema, weight loss, shortness of breath, palpitations, and feeling faint with standing. In AL amyloidosis, specific indicators can include enlargement of the tongue and periorbital purpura. In wild-type ATTR amyloidosis, non-cardiac symptoms include: bilateral carpal tunnel syndrome, lumbar spinal stenosis, biceps tendon rupture, small fiber neuropathy, and autonomic dysfunction.

Transthyretin (TTR or TBPA) is a transport protein in the plasma and cerebrospinal fluid that transports the thyroid hormone thyroxine (T4) and retinol to the liver. This is how transthyretin gained its name: transports thyroxine and retinol. The liver secretes TTR into the blood, and the choroid plexus secretes TTR into the cerebrospinal fluid.

Amyloid beta denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease. The peptides derive from the amyloid-beta precursor protein (APP), which is cleaved by beta secretase and gamma secretase to yield Aβ in a cholesterol-dependent process and substrate presentation. Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The oligomers are toxic to nerve cells. The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.

Familial amyloid polyneuropathy, also called transthyretin-related hereditary amyloidosis, transthyretin amyloidosis abbreviated also as ATTR, or Corino de Andrade's disease, is an autosomal dominant neurodegenerative disease. It is a form of amyloidosis, and was first identified and described by Portuguese neurologist Mário Corino da Costa Andrade, in 1952. FAP is distinct from senile systemic amyloidosis (SSA), which is not inherited, and which was determined to be the primary cause of death for 70% of supercentenarians who have been autopsied. FAP can be ameliorated by liver transplantation.

Cardiac amyloidosis is a subcategory of amyloidosis where there is depositing of the protein amyloid in the cardiac muscle and surrounding tissues. Amyloid, a misfolded and insoluble protein, can become a deposit in the heart's atria, valves, or ventricles. These deposits can cause thickening of different sections of the heart, leading to decreased cardiac function. The overall decrease in cardiac function leads to a plethora of symptoms. This multisystem disease was often misdiagnosed, with a corrected analysis only during autopsy. Advancements of technologies have increased earlier accuracy of diagnosis. Cardiac amyloidosis has multiple sub-types including light chain, familial, and senile. One of the most studied types is light chain cardiac amyloidosis. Prognosis depends on the extent of the deposits in the body and the type of amyloidosis. New treatment methods are actively being researched in regards to the treatment of heart failure and specific cardiac amyloidosis problems.

Alpha sheet is an atypical secondary structure in proteins, first proposed by Linus Pauling and Robert Corey in 1951. The hydrogen bonding pattern in an alpha sheet is similar to that of a beta sheet, but the orientation of the carbonyl and amino groups in the peptide bond units is distinctive; in a single strand, all the carbonyl groups are oriented in the same direction on one side of the pleat, and all the amino groups are oriented in the same direction on the opposite side of the sheet. Thus the alpha sheet accumulates an inherent separation of electrostatic charge, with one edge of the sheet exposing negatively charged carbonyl groups and the opposite edge exposing positively charged amino groups. Unlike the alpha helix and beta sheet, the alpha sheet configuration does not require all component amino acid residues to lie within a single region of dihedral angles; instead, the alpha sheet contains residues of alternating dihedrals in the traditional right-handed (αR) and left-handed (αL) helical regions of Ramachandran space. Although the alpha sheet is only rarely observed in natural protein structures, it has been speculated to play a role in amyloid disease and it was found to be a stable form for amyloidogenic proteins in molecular dynamics simulations. Alpha sheets have also been observed in X-ray crystallography structures of designed peptides.

In medicine, proteinopathy, or proteopathy, protein conformational disorder, or protein misfolding disease, is a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body. Often the proteins fail to fold into their normal configuration; in this misfolded state, the proteins can become toxic in some way or they can lose their normal function. The proteinopathies include such diseases as Creutzfeldt–Jakob disease and other prion diseases, Alzheimer's disease, Parkinson's disease, amyloidosis, multiple system atrophy, and a wide range of other disorders. The term proteopathy was first proposed in 2000 by Lary Walker and Harry LeVine.
Co-chaperones are proteins that assist chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly essential in stimulation of the ATPase activity of these chaperone proteins. There are a great number of different co-chaperones however based on their domain structure most of them fall into two groups: J-domain proteins and tetratricopeptide repeats (TPR).
The familial amyloid neuropathies are a rare group of autosomal dominant diseases wherein the autonomic nervous system and/or other nerves are compromised by protein aggregation and/or amyloid fibril formation.
Richard I. Morimoto is a Japanese American molecular biologist. He is the Bill and Gayle Cook Professor of Biology and Director of the Rice Institute for Biomedical Research at Northwestern University.

Tafamidis, sold under the brand names Vyndaqel and Vyndamax, is a medication used to delay disease progression in adults with certain forms of transthyretin amyloidosis. It can be used to treat both hereditary forms, familial amyloid cardiomyopathy and familial amyloid polyneuropathy, as well as wild-type transthyretin amyloidosis, which formerly was called senile systemic amyloidosis. It works by stabilizing the quaternary structure of the protein transthyretin. In people with transthyretin amyloidosis, transthyretin falls apart and forms clumps called (amyloid) that harm tissues including nerves and the heart.
Proteostasis is the dynamic regulation of a balanced, functional proteome. The proteostasis network includes competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking, and degradation of proteins present within and outside the cell. Loss of proteostasis is central to understanding the cause of diseases associated with excessive protein misfolding and degradation leading to loss-of-function phenotypes, as well as aggregation-associated degenerative disorders. Therapeutic restoration of proteostasis may treat or resolve these pathologies.
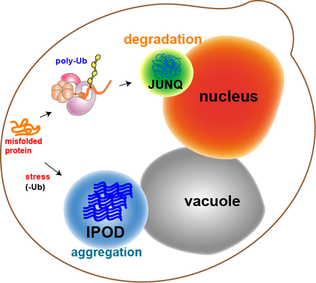
JUNQ and IPOD are types of cytosolic protein inclusion bodies in eukaryotes.
Chemical chaperones are a class of small molecules that function to enhance the folding and/or stability of proteins. Chemical chaperones are a broad and diverse group of molecules, and they can influence protein stability and polypeptide organization through a variety of mechanisms. Chemical chaperones are used for a range of applications, from production of recombinant proteins to treatment of protein misfolding in vivo.
Sheena Elizabeth Radford FRS FMedSci is a British biophysicist, and Astbury Professor of Biophysics in the Astbury Centre for Structural Molecular Biology, School of Molecular and Cellular Biology at the University of Leeds. Radford is the Associate Editor of the Journal of Molecular Biology.
Chaperome refers to the ensemble of all cellular molecular chaperone and co-chaperone proteins that assist protein folding of misfolded proteins or folding intermediates in order to ensure native protein folding and function, to antagonize aggregation-related proteotoxicity and ensuing protein loss-of-function or protein misfolding-diseases such as the neurodegenerative diseases Alzheimer's, Huntington's or Parkinson's disease, as well as to safeguard cellular proteostasis and proteome balance.

Michele Vendruscolo is an Italian British physicist working in the UK, noted for his theoretical and experimental work on protein folding, misfolding and aggregation.
Hilal Lashuel is an American-Yemeni neuroscientist and chemist, currently an associate professor at the EPFL. His research focuses on protein misfolding and aggregation in the pathogenesis of Alzheimer's and Parkinson's diseases.
References
- 1 2 3 Faculty biography, Scripps, Retrieved April 2, 2018
- 1 2 3 Borman, Stu (January 25, 2010). "Attacking Amyloids". Chemical & Engineering News. 88 (4): 30–32. doi:10.1021/cen-v088n004.p030.
- ↑ Timmerman, Luke (May 3, 2010). "Proteostasis, with San Diego Roots and Boston Home, Seeks Edge in Alzheimer's and Parkinson's". Xconomy.
- ↑ "Form S-1". Proteostasis Therapeutics via SEC Edgar. December 23, 2015.
- ↑ Preston, Juliet (February 5, 2013). "J&J Creates Space at Janssen Labs for Life Sciences Entrepreneurs". Xconomy.
- ↑ Labaudiniere, Richard (2014). "Chapter 9: Discovery and Development of Tafamidis for the Treatment of TTR Familial Amyloid Polyneuropathy". In Pryde, David C; Palmer, Michael J (eds.). Orphan Drugs and Rare Diseases. RSC Drug Discovery Series No. 38. Royal Society of Chemistry. ISBN 978-1-84973-806-4.
- 1 2 3 Jones, Dan (October 29, 2010). "Modifying protein misfolding". Nature Reviews Drug Discovery. 9 (11): 825–827. doi:10.1038/nrd3316.
- ↑ Breznitz, Shiri M.; O'Shea, Rory P.; Allen, Thomas J. (March 2008). "University Commercialization Strategies in the Development of Regional Bioclusters". Journal of Product Innovation Management. 25 (2): 129–142. doi:10.1111/j.1540-5885.2008.00290.x.
- ↑ see Commencement address at: youtu.be/4YA7btif6A8
- ↑ Wolf Prize in Chemistry 2023