
Ozone is an inorganic molecule with the chemical formula O
3. It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O
2, breaking down in the lower atmosphere to O
2 (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the latter, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation.

The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.

Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is referred to as the ozone hole. There are also springtime polar tropospheric ozone depletion events in addition to these stratospheric events.

The stratosphere is the second layer of the atmosphere of Earth, located above the troposphere and below the mesosphere. The stratosphere is an atmospheric layer composed of stratified temperature layers, with the warm layers of air high in the sky and the cool layers of air in the low sky, close to the planetary surface of the Earth. The increase of temperature with altitude is a result of the absorption of the Sun's ultraviolet (UV) radiation by the ozone layer. The temperature inversion is in contrast to the troposphere, near the Earth's surface, where temperature decreases with altitude.

Peroxyacetyl nitrate is a peroxyacyl nitrate. It is a secondary pollutant present in photochemical smog. It is thermally unstable and decomposes into peroxyethanoyl radicals and nitrogen dioxide gas. It is a lachrymatory substance, meaning that it irritates the lungs and eyes.

Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.

Chemiluminescence is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊,
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH3Cl. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant.

Mario José Molina Henríquez was a Mexican physical chemist. He played a pivotal role in the discovery of the Antarctic ozone hole, and was a co-recipient of the 1995 Nobel Prize in Chemistry for his role in discovering the threat to the Earth's ozone layer from chlorofluorocarbon (CFC) gases. He was the first Mexican-born scientist to receive a Nobel Prize in Chemistry and the third Mexican-born person to receive a Nobel prize.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, meteorology, computer modeling, oceanography, geology and volcanology and other disciplines. Research is increasingly connected with other areas of study such as climatology.

Paul Jozef Crutzen was a Dutch meteorologist and atmospheric chemist. He was awarded the Nobel Prize in Chemistry in 1995 for his work on atmospheric chemistry and specifically for his efforts in studying the formation and decomposition of atmospheric ozone. In addition to studying the ozone layer and climate change, he popularized the term Anthropocene to describe a proposed new epoch in the Quaternary period when human actions have a drastic effect on the Earth. He was also amongst the first few scientists to introduce the idea of a nuclear winter to describe the potential climatic effects stemming from large-scale atmospheric pollution including smoke from forest fires, industrial exhausts, and other sources like oil fires.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula Cl2FC−CClF2. This colorless, volatile liquid is a versatile solvent.
Paul O. Wennberg is the R. Stanton Avery Professor of Atmospheric Chemistry and Environmental Science and Engineering at the California Institute of Technology (Caltech). He is the director of the Ronald and Maxine Linde Center for Global Environmental Science. He is chair of the Total Carbon Column Observing Network and a founding member of the Orbiting Carbon Observatory project, which created NASA's first spacecraft for analysis of carbon dioxide in the atmosphere. He is also the principal investigator for the Mars Atmospheric Trace Molecule Occultation Spectrometer (MATMOS) to investigate trace gases in Mars's atmosphere.

SAGE III on ISS is the fourth generation of a series of NASA Earth-observing instruments, known as the Stratospheric Aerosol and Gas Experiment. The first SAGE III instrument was launched on a Russian Meteor-3M satellite. The recently revised SAGE III was mounted to the International Space Station where it uses the unique vantage point of ISS to make long-term measurements of ozone, aerosols, water vapor, and other gases in Earth's atmosphere.
Barbara J. Finlayson-Pitts is a Canadian-American atmospheric chemist. She is a professor in the chemistry department at the University of California, Irvine and is the Director of AirUCI Institute. Finlayson-Pitts and James N. Pitts, Jr. are the authors of Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications (1999). She has been a member of the National Academy of Sciences since 2006 and is the laureate for the 2017 Garvan–Olin Medal. In 2016 she co-chaired the National Academy of Science report "The Future of Atmospheric Chemistry Research"
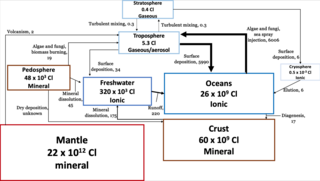
The chlorine cycle (Cl) is the biogeochemical cycling of chlorine through the atmosphere, hydrosphere, biosphere, and lithosphere. Chlorine is most commonly found as inorganic chloride ions, or a number of chlorinated organic forms. Over 5,000 biologically-produced chlorinated organics have been identified.
Anne Ritger Douglass is atmospheric physicist known for her research on chlorinated compounds and the ozone layer.

Bromine mononitrate is an inorganic compound, derived from bromine and nitric acid with the chemical formula BrNO3. The compound is a yellow liquid, decomposes at temperatures above 0 °C.














