
Gram stain, is a method of staining used to classify bacterial species into two large groups: gram-positive bacteria and gram-negative bacteria. It may also be used to diagnose a fungal infection. The name comes from the Danish bacteriologist Hans Christian Gram, who developed the technique in 1884.

Bacteriology is the branch and specialty of biology that studies the morphology, ecology, genetics and biochemistry of bacteria as well as many other aspects related to them. This subdivision of microbiology involves the identification, classification, and characterization of bacterial species. Because of the similarity of thinking and working with microorganisms other than bacteria, such as protozoa, fungi, and viruses, there has been a tendency for the field of bacteriology to extend as microbiology. The terms were formerly often used interchangeably. However, bacteriology can be classified as a distinct science.

Mycobacterium is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis and leprosy in humans. The Greek prefix myco- means 'fungus', alluding to this genus' mold-like colony surfaces. Since this genus has cell walls with a waxy lipid-rich outer layer containing high concentrations of mycolic acid, acid-fast staining is used to emphasize their resistance to acids, compared to other cell types.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.
The cell envelope comprises the inner cell membrane and the cell wall of a bacterium. In Gram-negative bacteria an outer membrane is also included. This envelope is not present in the Mollicutes where the cell wall is absent.
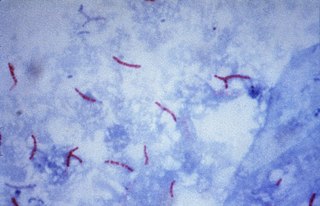
The Ziehl-Neelsen stain, also known as the acid-fast stain, is a bacteriological staining technique used in cytopathology and microbiology to identify acid-fast bacteria under microscopy, particularly members of the Mycobacterium genus. This staining method was initially introduced by Paul Ehrlich (1854–1915) and subsequently modified by the German bacteriologists Franz Ziehl (1859–1926) and Friedrich Neelsen (1854–1898) during the late 19th century.

Acid-fastness is a physical property of certain bacterial and eukaryotic cells, as well as some sub-cellular structures, specifically their resistance to decolorization by acids during laboratory staining procedures. Once stained as part of a sample, these organisms can resist the acid and/or ethanol-based decolorization procedures common in many staining protocols, hence the name acid-fast.
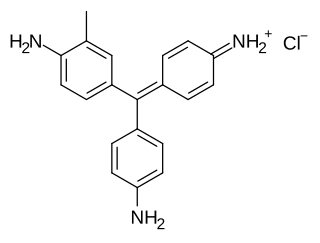
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl. There are other similar chemical formulations of products sold as fuchsine, and several dozen other synonyms of this molecule.

New fuchsine is an organic compound with the formula [(H2N(CH3)C6H3)3C]Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as single dyes. The other is pararosaniline. It is prepared by condensation of ortho-toluidine with formaldehyde. This process initially gives the benzhydrol 4,4'-bis(dimethylamino)benzhydrol, which is further condensed to give the leuco (colorless) tertiary alcohol [(H2N(CH3)C6H3)3COH, which is oxidized in acid to give the dye.

Mycobacterium smegmatis is an acid-fast bacterial species in the phylum Actinomycetota and the genus Mycobacterium. It is 3.0 to 5.0 μm long with a bacillus shape and can be stained by Ziehl–Neelsen method and the auramine-rhodamine fluorescent method. It was first reported in November 1884, who found a bacillus with the staining appearance of tubercle bacilli in syphilitic chancres. Subsequent to this, Alvarez and Tavel found organisms similar to that described by Lustgarten also in normal genital secretions (smegma). This organism was later named M. smegmatis.
Franz Ziehl was a German bacteriologist. He was a professor in Lübeck.

Carbol fuchsin, carbol-fuchsin, carbolfuchsin, or Castellani's paint is a mixture of phenol and basic fuchsin that is used in bacterial staining procedures. It is commonly used in the staining of mycobacteria because it has an affinity for the mycolic acids found in their cell membranes.

In staining dyes, nigrosin is a mixture of black synthetic dyes made by heating a mixture of nitrobenzene, aniline, and hydrochloric acid in the presence of copper or iron. Related to induline, it is a mixture of phenazine-based compounds. Its main industrial uses are as a colorant for lacquers and varnishes and in marker pen inks. Sulfonation of nigrosin yields a water-soluble anionic dye, nigrosin WS.
Auramine phenol stain is a stain used in clinical microbiology and histology to identify tuberculosis mycobacteria.

Acid fuchsin or fuchsine acid, (also called Acid Violet 19 and C.I. 42685) is an acidic magenta dye with the chemical formula C20H17N3Na2O9S3. It is a sodium sulfonate derivative of fuchsine. Acid fuchsin has wide use in histology, and is one of the dyes used in Masson's trichrome stain. This method is commonly used to stain cytoplasm and nuclei of tissue sections in the histology laboratory in order to distinguish muscle from collagen. The muscle stains red with the acid fuchsin, and the collagen is stained green or blue with Light Green SF yellowish or methyl blue. It can also be used to identify growing bacteria.

Löwenstein–Jensen medium, more commonly known as LJ medium, is a growth medium specially used for culture of Mycobacterium species, notably Mycobacterium tuberculosis.

Friedrich Carl Adolf Neelsen was a German pathologist.

Endospore staining is a technique used in bacteriology to identify the presence of endospores in a bacterial sample. Within bacteria, endospores are protective structures used to survive extreme conditions, including high temperatures making them highly resistant to chemicals. Endospores contain little or no ATP which indicates how dormant they can be. Endospores contain a tough outer coating made up of keratin which protects them from nucleic DNA as well as other adaptations. Endospores are able to regerminate into vegetative cells, which provides a protective nature that makes them difficult to stain using normal techniques such as simple staining and gram staining. Special techniques for endospore staining include the Schaeffer–Fulton stain and the Moeller stain.
In microbiology, the term isolation refers to the separation of a strain from a natural, mixed population of living microbes, as present in the environment, for example in water or soil, or from living beings with skin flora, oral flora or gut flora, in order to identify the microbe(s) of interest. Historically, the laboratory techniques of isolation first developed in the field of bacteriology and parasitology, before those in virology during the 20th century.

Mycobacterium ulcerans is a species of bacteria found in various aquatic environments. The bacteria can infect humans and some other animals, causing persistent open wounds called Buruli ulcer. M. ulcerans is closely related to Mycobacterium marinum, from which it evolved around one million years ago, and more distantly to the mycobacteria which cause tuberculosis and leprosy.















