Evolution is the change in the heritable characteristics of biological populations over successive generations. It occurs when evolutionary processes such as natural selection and genetic drift act on genetic variation, resulting in certain characteristics becoming more or less common within a population over successive generations. The process of evolution has given rise to biodiversity at every level of biological organisation.

Natural selection is the differential survival and reproduction of individuals due to differences in phenotype. It is a key mechanism of evolution, the change in the heritable traits characteristic of a population over generations. Charles Darwin popularised the term "natural selection", contrasting it with artificial selection, which is intentional, whereas natural selection is not.

In evolutionary biology, punctuated equilibrium is a theory that proposes that once a species appears in the fossil record, the population will become stable, showing little evolutionary change for most of its geological history. This state of little or no morphological change is called stasis. When significant evolutionary change occurs, the theory proposes that it is generally restricted to rare and geologically rapid events of branching speciation called cladogenesis. Cladogenesis is the process by which a species splits into two distinct species, rather than one species gradually transforming into another.

Sexual selection is a mechanism of evolution in which members of one biological sex choose mates of the other sex to mate with, and compete with members of the same sex for access to members of the opposite sex. These two forms of selection mean that some individuals have greater reproductive success than others within a population, for example because they are more attractive or prefer more attractive partners to produce offspring. Successful males benefit from frequent mating and monopolizing access to one or more fertile females. Females can maximise the return on the energy they invest in reproduction by selecting and mating with the best males.
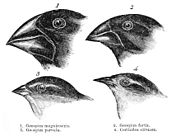
Speciation is the evolutionary process by which populations evolve to become distinct species. The biologist Orator F. Cook coined the term in 1906 for cladogenesis, the splitting of lineages, as opposed to anagenesis, phyletic evolution within lineages. Charles Darwin was the first to describe the role of natural selection in speciation in his 1859 book On the Origin of Species. He also identified sexual selection as a likely mechanism, but found it problematic.

The modern synthesis was the early 20th-century synthesis of Charles Darwin's theory of evolution and Gregor Mendel's ideas on heredity into a joint mathematical framework. Julian Huxley coined the term in his 1942 book, Evolution: The Modern Synthesis. The synthesis combined the ideas of natural selection, Mendelian genetics, and population genetics. It also related the broad-scale macroevolution seen by palaeontologists to the small-scale microevolution of local populations.

Evolutionary biology is the subfield of biology that studies the evolutionary processes that produced the diversity of life on Earth. It is also defined as the study of the history of life forms on Earth. Evolution holds that all species are related and gradually change over generations. In a population, the genetic variations affect the phenotypes of an organism. These changes in the phenotypes will be an advantage to some organisms, which will then be passed on to their offspring. Some examples of evolution in species over many generations are the peppered moth and flightless birds. In the 1930s, the discipline of evolutionary biology emerged through what Julian Huxley called the modern synthesis of understanding, from previously unrelated fields of biological research, such as genetics and ecology, systematics, and paleontology.

This is a list of topics in evolutionary biology.

Evolution of sexual reproduction describes how sexually reproducing animals, plants, fungi and protists could have evolved from a common ancestor that was a single-celled eukaryotic species. Sexual reproduction is widespread in eukaryotes, though a few eukaryotic species have secondarily lost the ability to reproduce sexually, such as Bdelloidea, and some plants and animals routinely reproduce asexually without entirely having lost sex. The evolution of sexual reproduction contains two related yet distinct themes: its origin and its maintenance. Bacteria and Archaea (prokaryotes) have processes that can transfer DNA from one cell to another, but it is unclear if these processes are evolutionarily related to sexual reproduction in Eukaryotes. In eukaryotes, true sexual reproduction by meiosis and cell fusion is thought to have arisen in the last eukaryotic common ancestor, possibly via several processes of varying success, and then to have persisted.

Genetics and the Origin of Species is a 1937 book by the Ukrainian-American evolutionary biologist Theodosius Dobzhansky. It is regarded as one of the most important works of modern synthesis and was one of the earliest. The book popularized the work of population genetics to other biologists and influenced their appreciation for the genetic basis of evolution. In his book, Dobzhansky applied the theoretical work of Sewall Wright (1889–1988) to the study of natural populations, allowing him to address evolutionary problems in a novel way during his time. Dobzhansky implements theories of mutation, natural selection, and speciation throughout his book to explain the habits of populations and the resulting effects on their genetic behavior. The book explains evolution in depth as a process over time that accounts for the diversity of all life on Earth. The study of evolution was present, but greatly neglected at the time. Dobzhansky illustrates that evolution regarding the origin and nature of species during this time in history was deemed mysterious, but had expanding potential for progress to be made in its field.
The gene-centered view of evolution, gene's eye view, gene selection theory, or selfish gene theory holds that adaptive evolution occurs through the differential survival of competing genes, increasing the allele frequency of those alleles whose phenotypic trait effects successfully promote their own propagation. The proponents of this viewpoint argue that, since heritable information is passed from generation to generation almost exclusively by DNA, natural selection and evolution are best considered from the perspective of genes.

In biology, altruism refers to behaviour by an individual that increases the fitness of another individual while decreasing their own. Altruism in this sense is different from the philosophical concept of altruism, in which an action would only be called "altruistic" if it was done with the conscious intention of helping another. In the behavioural sense, there is no such requirement. As such, it is not evaluated in moral terms—it is the consequences of an action for reproductive fitness that determine whether the action is considered altruistic, not the intentions, if any, with which the action is performed.
In physical attractiveness studies, averageness describes the physical beauty that results from averaging the facial features of people of the same gender and approximately the same age. The majority of averageness studies have focused on photographic overlay studies of human faces, in which images are morphed together. The term "average" is used strictly to denote the technical definition of the mathematical mean. An averaged face is not unremarkable, but is, in fact, quite good looking. Nor is it typical in the sense of common or frequently occurring in the population, though it appears familiar, and is typical in the sense that it is a good example of a face that is representative of the category of faces.
The Red Queen's hypothesis is a hypothesis in evolutionary biology proposed in 1973, that species must constantly adapt, evolve, and proliferate in order to survive while pitted against ever-evolving opposing species. The hypothesis was intended to explain the constant (age-independent) extinction probability as observed in the paleontological record caused by co-evolution between competing species; however, it has also been suggested that the Red Queen hypothesis explains the advantage of sexual reproduction at the level of individuals, and the positive correlation between speciation and extinction rates in most higher taxa.

Ecological speciation is a form of speciation arising from reproductive isolation that occurs due to an ecological factor that reduces or eliminates gene flow between two populations of a species. Ecological factors can include changes in the environmental conditions in which a species experiences, such as behavioral changes involving predation, predator avoidance, pollinator attraction, and foraging; as well as changes in mate choice due to sexual selection or communication systems. Ecologically-driven reproductive isolation under divergent natural selection leads to the formation of new species. This has been documented in many cases in nature and has been a major focus of research on speciation for the past few decades.
The rate of evolution is quantified as the speed of genetic or morphological change in a lineage over a period of time. The speed at which a molecular entity evolves is of considerable interest in evolutionary biology since determining the evolutionary rate is the first step in characterizing its evolution. Calculating rates of evolutionary change is also useful when studying phenotypic changes in phylogenetic comparative biology. In either case, it can be beneficial to consider and compare both genomic data and paleontological data, especially in regards to estimating the timing of divergence events and establishing geological time scales.

Social selection is a term used with varying meanings in biology.

The scientific study of speciation — how species evolve to become new species — began around the time of Charles Darwin in the middle of the 19th century. Many naturalists at the time recognized the relationship between biogeography and the evolution of species. The 20th century saw the growth of the field of speciation, with major contributors such as Ernst Mayr researching and documenting species' geographic patterns and relationships. The field grew in prominence with the modern evolutionary synthesis in the early part of that century. Since then, research on speciation has expanded immensely.

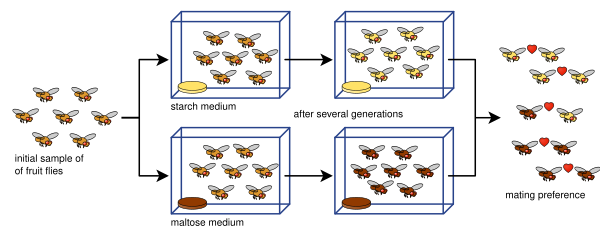
Laboratory experiments of speciation have been conducted for all four modes of speciation: allopatric, peripatric, parapatric, and sympatric; and various other processes involving speciation: hybridization, reinforcement, founder effects, among others. Most of the experiments have been done on flies, in particular Drosophila fruit flies. However, more recent studies have tested yeasts, fungi, and even viruses.
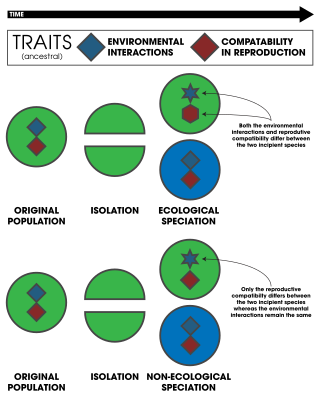
When speciation is not driven by divergent natural selection, it can be said to be nonecological, so as to distinguish it from the typical definition of ecological speciation: "It is useful to consider ecological speciation as its own form of species formation because it focuses on an explicit mechanism of speciation: namely divergent natural selection. There are numerous ways other than via divergent natural selection in which populations might become genetically differentiated and reproductively isolated." It is likely that many instances of nonecological speciation are allopatric, especially when the organisms in question are poor dispersers, however sympatric nonecological speciation may also be possible, especially when accompanied by an "instant" loss of reproductive compatibility, as when polyploidization happens. Other potential mechanisms for nonecological speciation include mutation-order speciation and changes in chirality in gastropods.